©Copyright 2018 GEOSCIENCE RESEARCH INSTITUTE
11060 Campus Street • Loma Linda, California 92350 • 909-558-4548

IRREDUCIBLE INTERDEPENDENCE: AN IC-LIKE ECOLOGICAL PROPERTY POTENTIALLY ILLUSTRATED BY THE NITROGEN CYCLE
by
Henry A Zuill
Professor of Biology (retired),
Union College, Lincoln Nebraska
and
Timothy G. Standish
Geoscience Research Institute
ABSTRACT
The nitrogen cycle is an ecochemical[1] pathway distributed on a global scale and including multiple organisms. Reactions comprising the nitrogen cycle are catalyzed by complex protein machines, some of which — like the nitrogen fixing system in legumes — may arguably be Irreducibly Complex (IC). The focus of this paper is not on these systems, but the overarching cycle in which they participate, asking if the cycle itself resembles an IC system, whether the components themselves are IC or not.
INTRODUCTION
Recent arguments for design have made use of information encoded in DNA and of irreducibly complex molecular machines. At the molecular level, enough knowledge has accrued to understand the nature and behavior of atoms and molecules with fair confidence. Thus when atoms are seen to be arranged in specific ways that are not required by their nature, and yet seem remarkably fortuitous, it seems reasonable to infer some kind of intelligent cause. Such is the case when atoms are arranged to encode information as in DNA, and also when atoms are arranged to form complex molecular machines or biochemical assembly lines.
In his groundbreaking book on the subject of Intelligent Design (ID),[2] Michael Behe popularized the term “irreducible complexity” (IC), and made the case that certain biochemical systems exhibit this property. Behe defined IC as:
A single system composed of several well-matched, interacting parts that contribute to the basic function, wherein the removal of any one of the parts causes the system to effectively cease functioning.[3]
Thus to understand whether a system is irreducibly complex (IC) requires that:
-
The function be known
-
It be composed of multiple interacting parts
-
These parts be well-matched
-
At least some subset of those parts be indispensable for the system to function at a minimal level (note that not all the parts must be indispensable)
The examples of IC biochemical systems Behe provides range from the machine-like bacterial flagellum to the complex cascade of biochemical events which occur to produce blood clots. Behe also discusses biosynthesis of Adenosine Mono Phosphate (AMP) which might not be irreducibly complex, but also presents problems for incremental construction.
All of Behe’s examples reside either within single cells, or at least within the same organism. Clearly this has profound implications if his thesis — that the presence of IC systems precludes a Darwinian explanation — is correct. But Behe did not restrict IC biochemical pathways and systems only to those found in a single organism. In fact, he encouraged examination of more complex systems to see if they exhibit IC-like properties:
Given that some biochemical systems were designed by an intelligent agent, and given the tools by which we came to that conclusion, how do we analyze other biochemical systems that may be more complicated and less discrete than the ones we have so far discussed?[4]
In this paper we attempt to address this question by arguing that the nitrogen cycle (N cycle) exhibits properties that resemble IC, but differ significantly from the examples used by Behe. Since the ecochemical nitrogen cycle is distributed across multiple species, and if Behe’s contention that IC precludes a Darwinian origin holds, the nitrogen cycle presents implications that go beyond those inherent in IC systems contained within a single organism.
THE NITROGEN CYCLE
The function of the N cycle is to regulate concentrations of various nitrogen-containing molecules in the environment in such a way that life can thrive. For those accustomed to thinking of the N cycle primarily in terms of nitrogen fixation for production of amino acids and other nitrogen containing molecules, this may seem counterintuitive. However, when viewed from a global perspective this is precisely what the N cycle achieves. In nature it works to keep reactive oxides of nitrogen, as well as chemically active reduced nitrogen compounds, particularly ammonia, at levels which allow life to exist while at the same time ensuring availability of reduced nitrogen when it is required for growth.
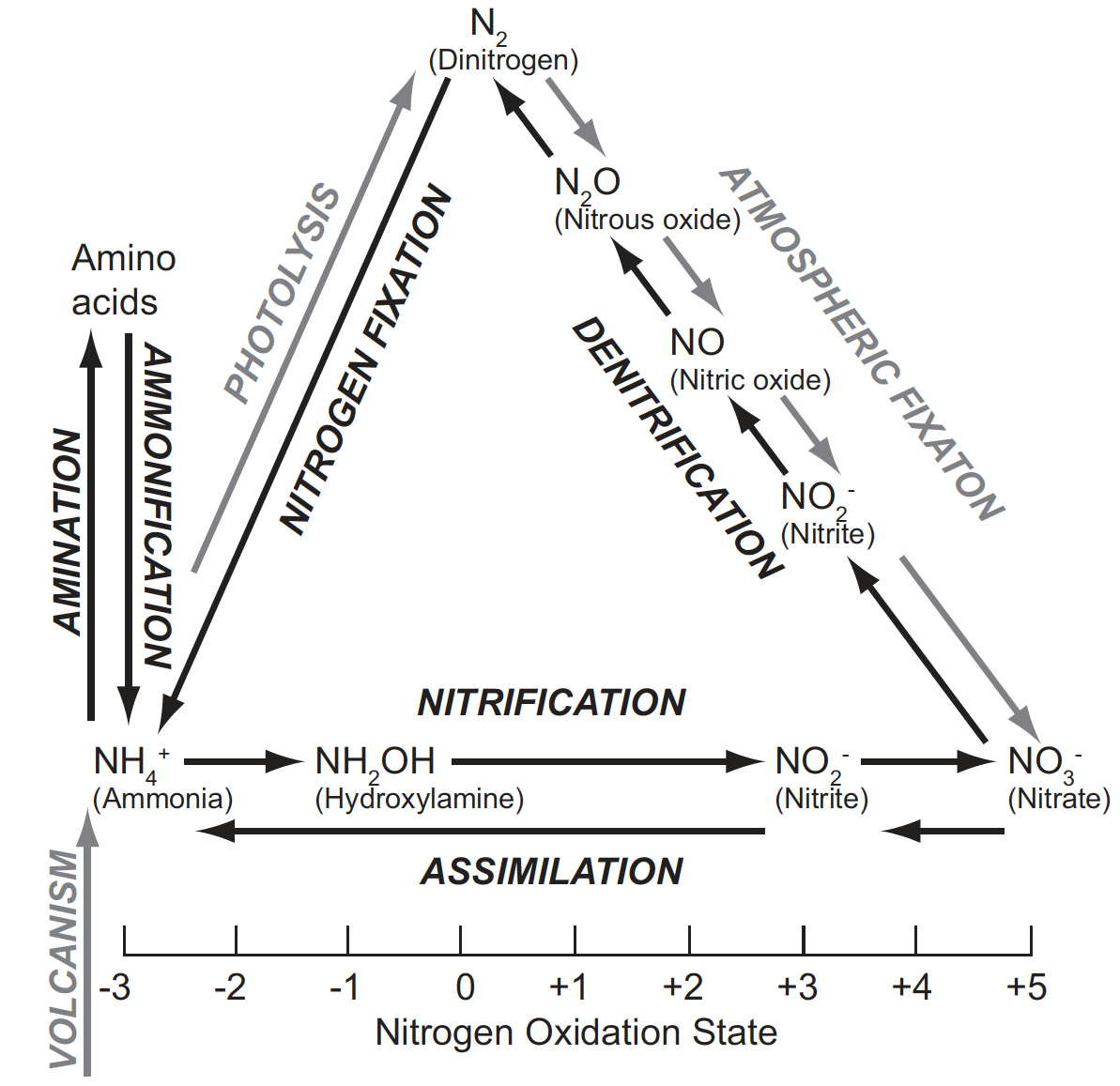
Figure 1. The Nitrogen Cycle. The nitrogen cycle involves a series of interconnected oxidation-reduction reactions. Of the major inorganic states in which nitrogen is found, the most common by far is as relatively inert atmospheric dinitrogen, followed by nitrate and ammonia whose relative abundance varies in different environments. Nitrogen in proteins and other organic nitrogen-containing molecules makes up another major repository of nitrogen. Other than nitrate, the various reactive nitrogen oxides shown are found in less abundance. Abiotic processes that mirror steps in the biological cycle are shown in gray. These abiotic processes contribute in relatively minor ways to maintaining the cycle and its global function of regulating abundance of various nitrogen-containing molecules. This figure is modified from Figure 1 in Cabello P. Roldán MD. Moreno-Vivián C. 2004. Nitrate reduction and the nitrogen cycle in archaea. Microbiology 150:3527-3546.
In essence, the N cycle functions to ensure that the vast majority of nitrogen atoms are in the form of the inert gas N2, while most of the remaining nitrogen is found in living things or their waste products. The cycle acts as a vital buffer to changes in nitrogen-containing molecules in the environment, while at the same time ensuring availability of reduced nitrogen for biological purposes. Some variation among different biomes on Earth is evident and some deviation from the current relative abundances of nitrogen in various chemical states may have occurred in the past, but life requires limits to the concentrations of various forms of nitrogen in the environment. It is the biological N cycle that prevents these limits from being exceeded under most circumstances. Because the ecological function of the N cycle is known, it meets Behe’s first requirement, that the function be known.
Figure 1 gives a typical depiction of the N cycle. It is clear that this cycle has multiple parts, thus fulfilling the second criterion laid down by Behe for a system to be IC. Whether these parts are “well-matched” is a matter of judgment. The bulk of the rest of this paper will examine two issues:
-
Whether some parts of the cycle are indispensable. By this we mean a part is necessary for the cycle to operate and lacking that step, the N cycle would not achieve its overall function.
-
Whether some reasonable step-by-small-step unguided natural process could be expected to produce the N cycle as we find it. In other words, can parts of the cycle be bridged by known inorganic processes in such a way that the cycle could be assembled incrementally as biological mechanisms accrued until the cycle became essentially a completely biological rather than abiotic process? Or are there necessary steps that are not practically bridgeable by inorganic processes?
In short, are the various stages of the nitrogen cycle indispensable to its function and do they represent functions that nature acting alone could not reasonably be expected to bridge?
FIVE STAGES OF THE NITROGEN CYCLE
The nitrogen cycle, sometimes said to be a web, consists of five stages: The first stage, Nitrogen Fixation, is the process by which atmospheric nitrogen is reduced to ammonia. This stage is particularly important and is made up of multiple sub-stages. The second stage, Nitrification, first converts ammonia to nitrite and then to nitrate. Another stage, Denitrification, changes nitrate back to either atmospheric dinitrogen or nitrous oxide, another gas. The fourth stage, Assimilation, converts nitrates back to nitrites and finally to ammonia. This ammonia is used to produce amino acids via amination and these amino acids are used to produce biological compounds such as proteins, or serve as substrates for production of other nitrogen-containing molecules including nucleic acids. The final stage in the cycle is Decay or ammonification (also known as mineralization), in which nitrogen from wastes and decaying organic nitrogenous residues are converted back to ammonia and then recycled. This process is usually slow, with most nitrogenous wastes remaining in soil as larger organic molecules (amino acids, for example, as well as protein fragments) which are slowly converted to ammonia. These amino acids and protein residues may even be directly absorbed by plants.[5]
Each stage in the nitrogen cycle involves specialized enzymes housed in widely diverse organisms. The nitrogen cycle, incorporating a broad spectrum of unconsciously cooperating species, operates in a coordinated assembly-line manner that is extraordinary and impressive. Whether it contains steps that are both indispensable and unbridgeable will be examined in the following sections of this paper.
1A. NITROGEN FIXATION — OVERVIEW
Nitrogen fixation occurs in one of three different ways, two of them natural: 1) Atmospheric (Lightning) Fixation, 2) Biological Fixation, and 3) Industrial Fixation (Haber Process), used for synthesizing fertilizers and explosives. In this paper, biological and atmospheric nitrogen fixation will be discussed, but industrial fixation will only be mentioned where it contributes to understanding the impact of unbalancing the natural nitrogen cycle.
Biological nitrogen fixation could be the subject of an entire design argument by itself, but for the purposes of this discussion the most important consideration is the final product: ammonia (NH3). Within cells, this reactive chemical must be handled with some degree of finesse if it is to react with the appropriate substrate and form an amino acid. It is these amino acid molecules which serve as nitrogen donors during synthesis of other nitrogen-containing organic molecules, like more complex amino acids and the nitrogen-containing bases of nucleotides.
1B. ATMOSPHERIC NITROGEN FIXATION
A relatively small, but not insignificant, amount of nitrogen is fixed by lightning passing through the atmosphere. Other phenomena, including thermal shock from meteorites striking the atmosphere, may have a similar effect. Thermal shock splits atmospheric dinitrogen molecules (N2), allowing the separated atoms to combine with oxygen, producing highly reactive nitrogen oxides which ultimately combine with water to form nitric acid (HNO3). Nitric acid is converted to nitrate in soils. Nitrates derived from atmospheric fixation mix with nitrates of biological origin and are assimilated by microbes or plants, or returned to the atmosphere as dinitrogen via denitrification.
1C. DOES ATMOSPHERIC NITROGEN FIXATION BRIDGE BIOLOGICAL FIXATION?
Because nitrates can be produced in the absence of biological nitrogen fixation, it might be tempting to suggest that this biological step in the nitrogen cycle is dispensable. In real life this is not the case because of three factors: 1) Nitrates from atmospheric fixation must be reduced to ammonia if they are to be biologically useful. 2) Electric storms and other causes of atmospheric fixation are more common in some places than others so nitrate produced by this means is irregularly distributed. 3) The amount of nitrogen fixed by thermal shock is comparatively small, so this method cannot be considered either consistent or sufficient in itself to sustain life as it is now.
One author has estimated (perhaps generously) that atmospheric nitrogen fixation produces as much as 10% of the total nitrogen fixed in nature.[6] Another reference[7] suggests that lightning fixes an estimated 3 to 5 Tg[8] annually, while annual bacterial fixation accounts for 90 to 130 Tg. Thus 10 % appears to be at the high end of estimates and the real percentage could very well be lower. A complicating factor is the contribution of agriculture, particularly intensive cultivation of legumes and rice, which has, over the past century, significantly increased biological nitrogen fixation on the continents. In the past, the contribution of atmospheric nitrogen fixation to total nitrogen fixation may have been higher as a percentage of the total, but the actual amount of nitrogen fixed in this way would be expected to remain relatively constant.
Atmospheric nitrogen fixation could not have been part of a bootstrap mechanism by which life originated because its product, nitrate, is not directly biologically useful. In addition, an abiotic mechanism to convert nitrate to biologically useful forms like ammonia is unavailable to bridge the gap between the products of atmospheric and biological fixation. There are no shared enzymes between biological nitrogen fixation and assimilation, even though their end product — ammonia — is the same. As a consequence, one cannot be explained as a relatively simple adaptation of the other to a different task.
In organisms living today, biological nitrogen fixation requires photosynthesis or chemosynthesis to provide both energy and carbon backbones for amination to produce amino acids. Of particular significance, both photosynthesis and chemosynthesis require nitrogen-containing proteins; thus, in these organisms a chicken-or-egg conundrum exists which atmospheric nitrogen fixation does not solve (Figure 2). In addition, during assimilation the reducing power may be provided by photorespiration[9]; thus a link exists between photosynthesis and both assimilation and nitrogen fixation.
How nitrates could have been abiotically modified to form biologically useful compounds is unclear. Even if the energy needed for nitrogen fixation or assimilation did not come from photosynthesis or chemosynthesis, some energy source is still required. In addition, enzymes that mediate the necessary reactions are also required. It may be possible to build a bypass around photosynthesis, but it is not clear that this would provide a more plausibly evolved pathway. No matter what the mechanism, complex protein catalysts appear to be required and production of these requires the ultimate products of nitrogen fixation — amino acids and nucleotides.
A further impediment to biological usefulness of atmospheric nitrogen fixation stems from the fact that nitrates form by reacting with oxygen. Nitrogen can exist in positive oxidation states between 1 and 5[10] (Figure 1). In general, nitrogen oxides are unstable and break down to form nitric oxide (NO) or nitrogen dioxide (NO2). Both of these oxides of nitrogen are highly reactive free radicals. NO2 constitutes the brown photochemical smog found in some cities, which serves as a catalyst in producing the potent oxidizer ozone (O3). Ozone oxidizes organic molecules and, if present in the low concentrations sufficient to destroy abiotically formed organic molecules, would hamper accumulation of the organic soup thought to be necessary for the “natural” origin of life. Therefore, the formation of nitrate as a result of atmospheric nitrogen fixation notwithstanding, life itself appears unlikely to have originated in an oxidizing atmosphere and lightning-induced nitrate production seems improbable as a source of biologically useful nitrogen during alleged evolution of nitrogen fixation systems. In an oxidizing atmosphere, life — if it already existed — must have possessed systems to deal with damage caused by toxic byproducts of atmospheric nitrogen fixation, but life is unlikely to have evolved in the first place due to the impact of some of these byproducts.
This may partly explain why, despite significant evidence to the contrary,[11] naturalistic “origin of life” scenarios commonly hinge on reducing primordial atmospheres.[12] Proposed atmospheres commonly contain gases such as ammonia, methane, hydrogen, and water vapor. Research involving atmospheres consisting of various combinations of these gases, but always lacking oxygen, have been shown, when supplied with sufficient energy, to produce a variety of organic molecules including amino acids. Thus, under reducing conditions, early life could freely acquire amino acids without resorting to biological nitrogen fixation. The problem is that, while this scenario might explain why amino acids serve as nitrogen donors in anabolic biochemical pathways, it still does not explain evolution of the nitrogen cycle itself; at best it renders one step in the cycle superfluous while necessitating evolution of other steps to cycle nitrogen out of organic molecules and back into the atmosphere. In any case, the problems of biochemical evolution and the spontaneous generation of life have been so much discussed that there is no need to repeat them. For an overview, see the chapter on the Miller-Urey experiment in Jonathan Well’s Icons of Evolution.[13]
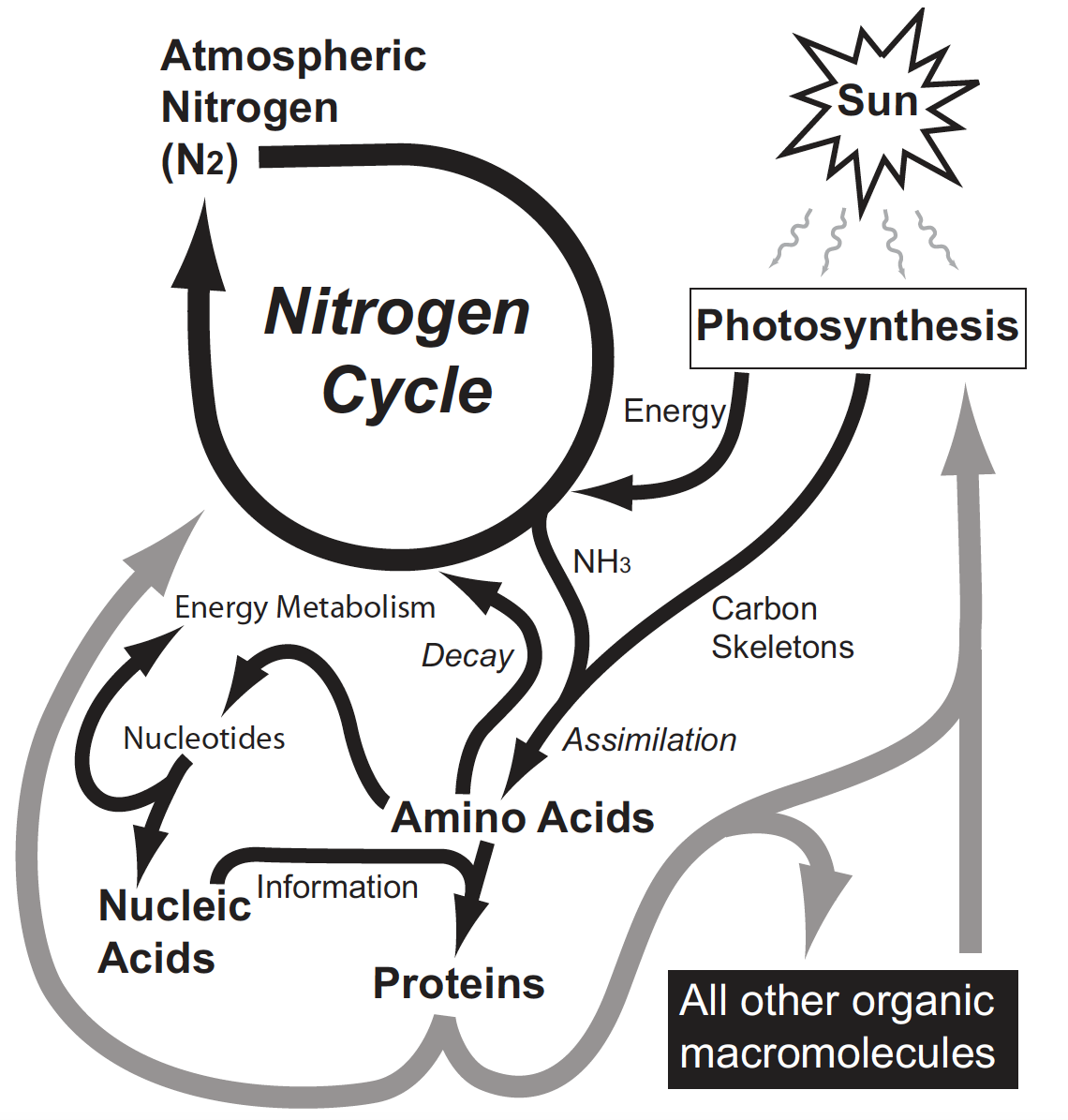
Figure 2. Ecology of the Nitrogen Cycle. The nitrogen cycle requirse atmospheric nitrogen, an energy source (typically photosynthesis), and enzymatic facilitation. Photosynthesis also provides carbon skeletons for amino acids which are aminated using nitrogen fixed in the nitrogen cycle. These amino acids serve in turn as building blocks of the enzymes and other proteins involved in both photosynthesis and the nitrogen cycle. In addition, amino acids provide the nitrogen found in nucleotides which are central to energy metabolism and serve as the building blocks of both DNA and RNA. Ultimately, protein enzymes mediate the manufacture of all biological macromolecules. Thus, all the vital processes found in living things are interdependently linked via the nitrogen cycle. Note that assimilation and decay are really part of the nitrogen cycle, but for clarity, these processes have been identified separately in this illustration.
Most arguments for evolution of the nitrogen cycle allow for the existence of life before a complete nitrogen cycle existed, but some source of nitrogen in the right form is required for life to exist. This is a major problem. If a reducing atmosphere provides the nitrogen-containing building blocks of life, then biological nitrogen fixation becomes unnecessary raising the question of — at least before the switch from a reducing to an oxidizing atmosphere — what selective pressure would “cause” it to evolve. On the other hand, if nitrate is produced via thermal shock in an oxidizing atmosphere, then some unknown abiotic mechanism must have reduced the nitrate to a biologically useful form before evolution of mechanisms of assimilation. In addition, any reduced organic molecules must be protected in some way from O3 and other free radicals produced as a byproduct of atmospheric fixation. In either scenario, production of life and evolution of biological nitrogen fixation present conundrums that the neo-Darwinian mechanism does not reasonably resolve.
While any number of scenarios may be suggested to overcome these issues, none actually solves the problems using strictly Darwinian principles. Take the following scenario for example: life evolves in a reducing atmosphere which subsequently changes to an oxidizing atmosphere. Under these new circumstances, bacteria among the few organisms that survived the change evolve the ability to use nitrogen in nitrate thus evolving assimilation before biological nitrogen fixation. Life is sustained by atmospheric fixation until biological nitrogen fixation evolves. Problems with this scenario include: 1) It assumes that assimilation is evolvable and had evolved enough before it was vital to sustain some bacteria that also had the ability to survive an oxidizing atmosphere; 2) it assumes atmospheric fixation at levels sufficient to sustain life, but not so rapid that nitrate accumulated to the point that it caused problems; 3) evidence is lacking for a reducing atmosphere; 4) the concurrent need to develop a means of aminating carbon skeletons to produce amino acids; 5) the concurrent need to deal with radicals produced as part of the process; 6) availability of energy resources and reducing power sufficient to allow assimilation to work and so on. Probably the most troubling assumption is that any organism adapted to living in a reducing environment could survive the transition to an oxidizing environment. Ultimately scenarios of this kind simply split a single big problem into two big problems for Darwinism to explain; they do not reduce the problem to small steps that unguided nature might reasonably be expected to take via the neo-Darwinian process. In addition, they do not explain biological nitrogen fixation, but instead invoke a different biological means of obtaining nitrogen without addressing the point about nitrogen fixation. Assimilation will be further discussed later in this paper.
1D. BIOLOGICAL NITROGEN FIXATION: NITROGEN MADE AVAILABLE IN MANY HABITATS
Biological nitrogen fixation is the main natural[14] method by which nitrogen is made available to living organisms. As already noted, in natural systems over 90 percent of fixed nitrogen comes from biological activity. The ability to fix nitrogen is restricted to certain microbes. Bacteria (including cyanobacteria) that reduce nitrogen to ammonia (NH3) span a selection of widely disparate genera and lifestyles, examples of which include: Azotobacter (aerobic), Klebsiella (facultatively anaerobic), Rhodospirillum (photosynthetic, anaerobic), Clostridium (free-living/anaerobic), Nostoc (free living or symbiotic cyanobacterium), Frankia (actinomycete, symbiotic with Alnus, alder trees), Anabaena (photosynthetic cyanobacterium, symbiotic with Azolla, water fern; reported as common in rice paddies),[15] and Rhizobium (symbiotic with legumes). The latter four genera form symbiotic relationships with several genera of plants, although some species may also be free-living. While several other examples are known,[16] the best understood of such mutualistic relationship is that of Rhizobium strains and species in relationship with different legume species.
Anaerobic nitrogen-fixing bacteria are found in the guts of some herbivores including sea urchins[17] and termites.[18] The contribution of these bacteria to the nitrogen needs of their host may be negligible in some cases, but significant in others. Cyanobacteria may form symbiotic relationships (in lichens, for example), but it is as free-living organisms in aquatic and marine environments that they are especially important. Trichodesmiumis one such marine nitrogen-fixing cyanobacterium.[19]
The diversity of nitrogen-fixing bacteria ensures that nitrogen is made available to occupants of many different habitats. In addition, it illustrates the argument in this paper that the nitrogen cycle is not so much about individual species, but about steps in an ecochemical pathway. A step may be necessary and unbridgeable, but an individual species that mediates the step may not be necessary at a given time as the machinery required to accomplish the step — the enzymes involved — may be found in other species, some apparently distantly if at all related. Redundancy is important as a back-up when circumstances preclude the presence or sufficient abundance of individual species that have the same abilities. Ecological systems are replete with redundancies.
1E. BIOLOGICAL NITROGEN FIXATION — NITROGENASE
All known nitrogen-fixing bacteria produce nitrogenase, which is composed of two different protein complexes whose amino acids contain nitrogen. The existence of these protein complexes requires the very reactions they catalyze. When two different nitrogenase subunits from unrelated species are combined, they most often form “active hybrids” with nitrogenase activity.[20] Consequently, nitrogenases from even very distinct species appear comparable, although some differences have been noted.[21] This degree of similarity suggests a similar origin even though, as already noted, nitrogen-fixing bacteria occupy a range of very different habitats. Under these circumstances convergent evolution appears unlikely to have produced similar protein complexes capable of interchanging parts. Lateral gene transfer may represent the most promising evolutionary explanation of the distribution of nitrogenase across species.[22]
Nitrogenase expression is reversibly regulated by what is called the “ammonia switch-off.”[23] In addition, nitrogenase expression may be repressed via a complex cascade of events when oxygen levels are high.[24] While nitrogenase complexes in different species appear comparable, genetic regulation of nitrogenase expression differs widely in different organisms.[25] In addition, strategies for shielding nitrogenase from oxygen vary among organisms.
Interactions between host plants and Rhizobium bacteria in root nodules are particularly intimate and elegant.[26] When concentrations of nitrogen compounds are elevated in the shoots of host-plants, nitrogenase activity is lowered. Evidently, when no more fixed nitrogen is needed there is a means of communication between the host plant’s shoots and bacteroids, misshapen Rhizobium cells in root nodules.[27] This is another example of interspecific cooperation, which in this case is believed to involve an amino acid as the inhibitor of nitrogenase.[28] Down regulation of nitrogenase is necessary due to its high energy demands and the reactive nature of its product, ammonia. Under normal conditions, free ammonia is essentially absent as it is immediately used to produce the amino acid glutamate and is thus sequestered in a glutamate pool.
Significantly, in all known cases oxygen acts as a poison to the nitrogenase enzyme. If nitrogen fixation had evolved in a reducing atmosphere, this may make some sense, but a reducing atmosphere should eliminate the need for nitrogen fixation as nitrogen would be freely available via abiotically produced amino acids and as ammonia. Thus, selective pressure for developing nitrogen fixation is difficult to conceive, especially given its high energy demands. As a consequence, the sensitivity of nitrogenase to oxygen presents a conundrum; in a reducing atmosphere, nitrogen fixation should not evolve, while in an oxidizing environment nitrogenase does not work.
Invoking a neutral atmosphere to circumvent this problem does not solve it and presents the worst of both options. On the one hand, neutral atmospheres are not known to produce nitrogen-containing molecules essential for life and on the other hand, oxygen may still be present in concentrations sufficient to poison nitrogenase. Under these circumstances, nitrogen fixation would need to evolve for life to exist before life could exist, a veritable evolutionary “Catch 22.” In addition, some mechanism for isolating nitrogenase would still need to evolve to protect it from the relatively low levels of oxygen present in such an atmosphere. A simpler and more direct path would be to evolve a nitrogenase that is not as sensitive to oxygen. Clearly the sensitivity of nitrogenase to oxygen is not well explained by invoking its evolution in a reducing atmosphere or in a neutral one. This suggests that there may be a necessary design constraint that is worth looking for in nitrogenase, as that may be the true explanation of its sensitivity to oxygen.
All organisms that fix nitrogen use some mechanism to ensure anaerobic conditions. A notable example of this is leghaemoglobin, which occurs in legume root nodules and has greater affinity for oxygen than mammalian hemoglobin. Leghaemoglobin is cooperatively manufactured, with legume genes determining the globin portion of the molecule, while the porphyrin ring comes from Rhizobium.[29] However, the central iron ion in the porphyrin ring comes from the plant. Clearly, production of leghemoglobin requires exact coordination between both species. Cooperative synthesis, such as this, challenges Darwinian explanations and is another possible example of a system with IC-like characteristics spread across multiple species.
Most biological fixation is accomplished by symbiotic bacteria and photosynthetic nitrogen-fixing cyanobacteria.[30] Nitrogen fixation in freeliving non-photosynthetic soil bacteria is considered to be relatively low as a result of limited access to energy resources. Consequently, populations of such bacteria are also low.[31] However, they may be more numerous and productive close to roots, a zone designated as the “rhizosphere,” where they may access photosynthetically produced nutrient exudates. Nevertheless, in the words of Moat & Foster: “Although free-living organisms, in general, appear less efficient in their ability to fix nitrogen, their number, variety, and ubiquitous distribution suggest that they are of major ecological importance.”[32]
1F. BIOLOGICAL NITROGEN FIXATION AND PHOTOSYNTHESIS
Biological nitrogen fixation requires hydrogen and large amounts of energy from ATP. The reaction is represented in the following equation:
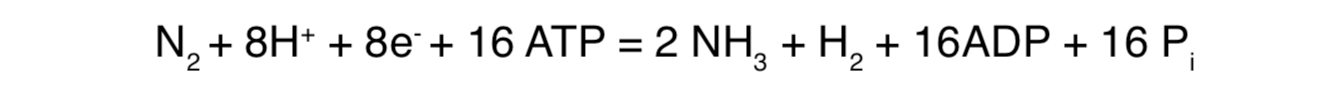
Notably absent is a stepwise chemical reduction in which oxides of nitrogen are used as intermediates in a biochemical pathway to nitrogen fixation. This precludes the pathway used in assimilation for reduction of nitrates as a stepping stone toward evolution of nitrogen fixation as observed today. Instead, nitrogenase-catalyzed reduction of N2 involves this complex protein machine directly transferring electrons to N2 in stepwise fashion.[33] 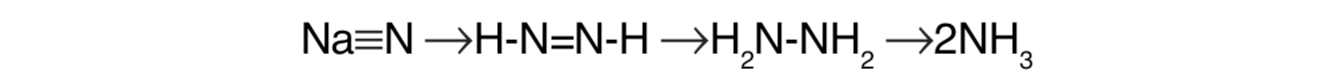
Improbable as it may seem, the sum of the Gibbs free energy (G) in these reactions is -79.0 kJ/mol.[34] In other words, the conversion of N2 to ammonia is exergonic. Among other things, the need for energy stems from the cost of providing hydrogen and electrons to the reaction, and that energy is derived from ATP which is either directly or indirectly produced by photosynthesis or, rarely, chemosynthesis. Moat & Foster[35] note that the photosynthetic capacity of plants may be a limiting factor in nitrogen fixation. It is estimated that as much as 20% of ATP produced in photosynthesis may be used for nitrogen fixation.[36] In legumes, fixing 1 mg of nitrogen require 4 mg of fixed carbon from the host plant.[37] Clearly, there is a necessary relationship between photosynthesis or chemosynthesis to supply energy for biological nitrogen fixation with its large energy requirement. In addition, ATP, contains a nitrogenous base, with its nitrogen traceable directly back to the nitrogen cycle.
Symbiotic rhizobia have direct access to chemical energy from the host-plant’s photosynthesis, but free-living bacteria depend upon such energy either provided by their own photosynthetic processes (cyanobacteria), or if non-photosynthetic, from respiration or fermentation of photosynthetically derived reduced organic molecules absorbed from soil, mostly in the rhizosphere. Thus, relationships in the nitrogen cycle appear complex and obligatory, even for free-living species.
1G. IS BIOLOGICAL NITROGEN FIXATION INDISPENSABLE AND UNBRIDGEABLE?
Unquestionably, biological nitrogen fixation is no simple process and a design argument could be made based on this single step in the nitrogen cycle. It is unlikely to have been produced via a step-by-step Darwinian process because nitrogenase itself is immensely complex, requires auxiliary complex mechanisms to maintain low oxygen tension, and also needs reduced carbon backbones as substrates for amination to store ammonia as glutamine. In addition, regulatory mechanisms are needed to coordinate the entire energetically expensive activity and its chemically reactive product, ammonia.
Of equal importance to asking if biological nitrogen fixation could be produced in some gradual manner is the question of whether known natural abiotic processes — like atmospheric nitrogen fixation — could bridge or by-pass this step in the cycle. As already discussed, the answer in the case of atmospheric fixation is that the product — nitrate — is not directly useful and the chemical intermediates in nitrate production are destructive to organic molecules as is nitrate itself when in the form of nitric acid. Assimilation of nitrate requires a separate photosynthesis-dependent mechanism, at least in plants, which would be unlikely to develop in the absence of nitrogen-containing proteins.
A more promising inorganic work around might be ammonia released by volcanoes, but volcanoes today do not release ammonia in large quantities.[38] Even if they did, a secondary problem results from the fact that ammonia is readily subject to photolysis. The high solubility of ammonia in water may protect some ammonia from being broken down by light, but significant quantities of ammonia in water would raise the pH impacting water chemistry in a way that presents challenges for life. Whatever the abiotc source of ammonia, whether from volcanoes, a reducing atmosphere or some other source, none serves as a probable natural bridge over biological nitrogen fixation as, when nature provides nitrogen for free in the form of ammonia or amino acids, selective pressure for an energy hungry metabolic process like nitrogen fixation seems unlikely.
2A. NITRIFICATION
Some ammonia produced in nitrogen fixation, as well as in ammonification (yet to be discussed), is directly taken up by plants through their roots, or from root-nodules, and assimilated, but large quantities of ammonia are also converted to nitrite and nitrate, a process generally known as nitrification. Many plants appear to preferentially take up nitrogen as nitrate (NO3 -). However, under conditions that are unfavorable for nitrification (low pH, anaerobic soils, etc), plants use ammonia. Use of ammonia as a primary source of nitrogen tends to lower soil pH.[39] But even under unfavorable conditions, nitrification still occurs at a relatively slower rate.[40] Aquatic plants absorb ammonia through their leaves.
Organisms (largely bacteria) that convert ammonia to nitrites and nitrates are referred to as nitrifiers. They are found in a variety of environments — soils, seawater, brackish waters, rivers, lakes, and waste water treatment ponds, etc. Along with some other genera, Nitrosomonas converts ammonia to nitrite (NO2 -). In general, organisms that only oxidize to nitrite are referred to as ammonia oxidizers. Nitrite itself is quickly oxidized so little of it is available to be absorbed by plants. Since nitrite is toxic, its rapid conversion to nitrate detoxifies while benefiting both organisms that absorb nitrates and bacteria that reap energy in the process.
Nitrobacter, along with several other genera, oxidizes nitrite to nitrate.[41] All nitrifiers are aerobic and most are chemoautotrophic, the energy derived from nitrification is used to fix carbon. A few nitrifiers are heterotrophic. For example, in forest litter, it is not bacteria, but saprophytic fungi, which do most of the nitrifying.[42]
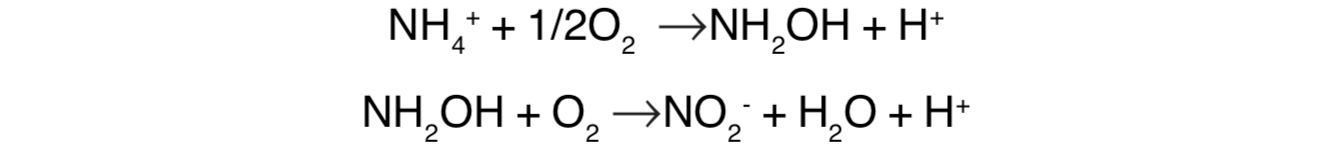
Nitrification is a two-step process, as already indicated. The first step, using the enzyme ammonia monoxygenase, is given in the following equations:
this initial nitrification reaction, 66 kcal of energy are liberated per mole of ammonia oxidized. Under oxygen limited conditions, the product is N2O (nitrous oxide) instead of nitrite.
The second step is as follows:
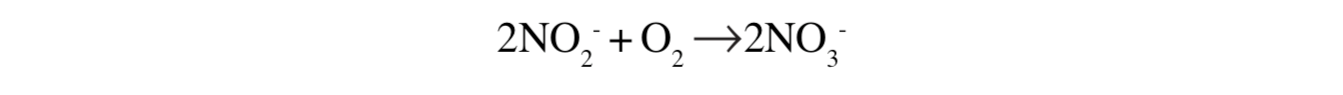
This step liberates 18 kcal per mole of nitrite oxidized.
Why is nitrification essential to the nitrogen cycle when plants and bacteria are able to use ammonia directly? Indeed, even nitrate must be reduced back to ammonia before it becomes biologically accessible. That some organisms even have the enzyme system that enables them to use nitrate when the simpler alternative to use ammonia directly is available, says much about the evident importance of the more roundabout route through nitrate.
As chemoautotrophs, nitrifiers fix carbon and make it available to respiration. However, the process is not very efficient. A more reasonable answer is suggested in defining the function of the nitrogen cycle as it was earlier in this paper: “to regulate concentrations of various nitrogencontaining molecules in the environment in such a way that life can thrive.” For three reasons, conversion of ammonia to nitrate is an essential part of the cycle’s function of regulating various nitrogen-containing molecules:
- It prevents accumulation of ammonia to toxic levels
- It provides a biologically available, but relatively chemically inert reservoir of nitrogen that can be utilized without requiring the complex and energetically expensive mechanisms used in biological nitrogen fixation
- The solubility of nitrate in water allows it to be relatively mobile, thus distributing biologically available nitrogen to organisms that do not have the ability to fix their own nitrogen.
Nitrification is thus an essential step in recycling nitrogen back to the atmosphere and plays a vital role in the global function of the nitrogen cycle in regulating nitrogen-containing molecules in the environment. It is worth noting that this understanding of the role and necessity of nitrification is driven by a design-oriented view of the nitrogen cycle and not a reductionistic view of nature.
2B. IS NITRIFICATION INDISPENSABLE AND UNBRIDGEABLE?
Total nitrogen in the atmosphere amounts to approximately 3.85 × 1021g.[43] It has been estimated that before significant human involvement in the process, biological nitrogen fixation amounted to 90-140 Tg per year.[44] Under these rates of fixation, all atmospheric nitrogen would theoretically be fixed as ammonia within approximately 27 to 43 million years. This estimate does not include the relatively small amount of nitrogen fixed in the past via thermal shock, which would shorten the time somewhat. Because current rates of nitrogen fixation are significantly higher due to intensive agriculture of legumes, industrial fixation and industrial combustion among other factors, the number of years at today’s rate would be considerably less. But this time span should not be understood as literally true because other factors like dissolved nitrogen in water and ammonia photolysis are not taken into consideration. What estimates like this do show is that the Earth could be expected to become thoroughly unfit for life due to ammonia accumulation in a time span considered short from a Darwinian perspective. This illustrates the necessity of a reverse pathway for removal of excess nitrogen. To the extent that nitrification is a step in this process, it is indispensable.
How might a process like nitrification come about by Darwinian selection or be naturally bridged? In a reducing environment in which nitrogen fixation is not necessary, the reverse process might appear to be unnecessary as well. However, this seems unlikely; nitrogen incorporated into organisms would still need to be recycled when excreted as a waste product or following death. But this might be accomplished by pathways in which nitrogen could be released from amino acids. For example, if nitrogen from amino acids was recycled back into ammonia, as occurs with deamination of glutamate by glutamate dehydrogenase, this would prevent infinite accumulation of amino acids. Whatever the mechanism, in a reducing environment it seems unlikely that “nitrification” would have evolved to be anything like the oxidative process of nitrification seen today.
An oxidizing atmosphere presents an interesting situation. Ammonia in the presence of oxygen burns readily, producing nitrogen oxides and water. In addition, at even relatively low concentrations, ammonia is toxic to life. In the absence of enzymes in living things and at low concentrations, ammonia does not spontaneously oxidize to nitrogen oxides and water at a significant rate. In an oxidizing atmosphere, without nitrification, ammonia would be expected to accumulate in the environment until one of two (possibly both) things happened:
- Equilibrium between organic ammonic production and inorganic ammonia degradation was reached, potentially resulting in ammonia concentrations incompatible with life.
- Catastrophic oxidation set off by lightning or some other spark occurred.
The latter scenario is improbable given the solubility of ammonia in water. More reasonably, ammonia would be expected to accumulate in bodies of water turning them basic. This assumes that photolysis of ammonia in the atmosphere does not break down ammonia fast enough to preclude its accumulation. In our present world, neither of these scenarios occurs because nitrification limits accumulation of ammonia, but allows for a ready supply of nitrogen to organisms in the relatively inert form of nitrate.
To get around problems resulting from the absence of nitrification, ammonia might be recycled into living material as it is in forests until some other limiting nutrient prevented further growth. As organisms died and the other limiting nutrient was recycled, biomass might be expected to accumulate until some conflagration burns all the accumulated nitrogencontaining biomass, returning the nitrogen to the atmosphere as nitrogen oxides. Nitric oxide (NO) and nitrogen dioxide (NO2) are both highly reactive gases dangerous to life. Thus it would be expected that biomass would accumulate past some tipping point and, at least on a local scale, destroy life. Nitrification prevents this kind of scenario by shuttling nitrogen in excess ammonia to a relatively benign molecule (nitrate) that can still be used by plants or, alternatively, continue on into denitrification where it is returned to the atmosphere as safe and inert N2.
3A. DENITRIFICATION[45]
Denitrification is a microbial respiratory process by which nitrate is reduced to atmospheric dinitrogen gas (N2) or nitrous oxide (N2O). Without this process, nitrates would accumulate in high concentrations, as has been seen in recent years with the overuse of nitrogenous fertilizers. On a global scale, in the absence of denitrification and sufficiently rapid assimilation by plants and microbes, nitrates would accrue in and acidify bodiesof water while the concentration of atmospheric nitrogen would decline. In fact a mechanism similar to this has been proposed to explain the unexpectedly low nitrogen concentration in the Martian atmosphere.[46] As it is, under normal conditions on Earth, nitrogen is often limiting in the biosphere as a result of low levels of nitrogen fixation along with denitrification.[47]
Organisms in soils require oxygen, but if soils are waterlogged for protracted periods (greater than 36 hours) and water fills spaces between soil particles usually occupied by air, then oxygen will be excluded. At such times, certain microbes are able to obtain essential oxygen from nitrite and nitrate. The oxygen from nitrate serves as an alternative electron acceptor.[48] The process is given in the following equation:
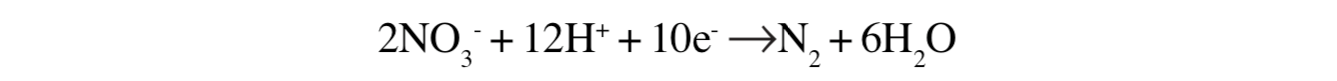
Another way of representing the process is:
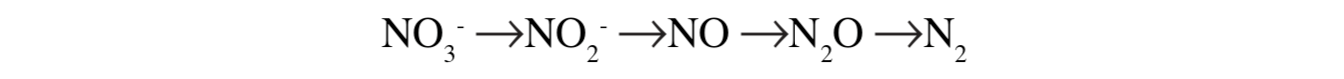
The last two products, nitrous oxide and dinitrogen, are returned to the atmosphere. Factors influencing denitrification include: the quantity of organic material available, waterlogging and oxygen deprivation, soil temperature, levels of soil nitrates and pH. For example, denitrification is higher during summer when water temperatures are highest.
Under normal conditions, waterlogging induces denitrification, which occurs at a rate amenable to environmental wellbeing. But when there is a nitrate overload, the highest attainable rates of denitrification may not be able to keep pace with demand and thus, nitrates may be carried to the water table and into aquifers. The result is eutrification of surface waters in which organisms grow so rapidly that oxygen is depleted resulting in death of many organisms. Ultimately, this may lead to increased rates of denitrification if nitrate becomes the most abundant electron acceptor available. Thus, even when the system is perturbed, it may be designed to still work to rectify the perturbation.
3B. IS DENITRIFICATION INDISPENSABLE AND UNBRIDGEABLE?
The necessity of denitrification is evident when the logic applied to nitrification is also applied to this step. While nitrates can be recycled into plant material, the heterogeneity of nature and lack of rapid transport mechanisms for nitrate ensure that concentrations would, at least locally, reach high levels. While nitrate is relatively immobile in the absence of water, it is water soluble and can be leached out into bodies of water where it may reach significant concentrations. Excess nitrates have the potential to cause environmental damage as evidenced in the consequences of over-use of industrially fixed nitrogen for agricultural purposes. Under current conditions, if denitrification was not part of the nitrogen cycle, even under the natural rates of nitrogen fixation and nitrification, nitrate levels could be expected to eventually become excessive.
Compared to other nitrogen oxides, nitrate is relatively stable and does not spontaneously degrade at an appreciable rate to O2 and N2 or N2O. In an oxidizing atmosphere, nitrates are produced via atmospheric fixation with lightning providing a significant portion of the energy driving the reaction. At current rates, approximately 3 to 5 × 1012 g of nitrogen are fixed per year[49] as nitrate via atmospheric fixation, meaning that, in the absence of biological nitrogen fixation and denitrification, all atmospheric nitrogen would theoretically be fixed as nitrogen oxides in approximately 1 billion years.[50] Again, this number is meant to be illustrative rather than literal, as it does not take into consideration reverse reactions and the impact of reduced nitrogen and oxygen concentrations in the atmosphere among other factors. In addition, this only takes into consideration abiotic processes. If biological nitrification was occurring, accumulation would be significantly faster. Assimilation does not act as a realistic way of removing nitrate as it simply recycles it into plants. As long as biological nitrogen fixation feeds nitrogen from the atmosphere into the nitrogen cycle, a way of removing nitrogen is necessary.
In the absence of biological denitrification, nitrate would be expected to accumulate. This is exactly what occurs in the Atacama Desert in northern Chile, which is among the driest areas on Earth.[51] Average annual rainfall is between 1 and 2 mm. In addition, when rain does fall, it drains away rapidly as there are no soils as such to become waterlogged. In this arid region, conditions necessary for denitrification rarely occur. It is thus not surprising that, as in several other deserts, nitrate has accumulated. But unlike other deserts, this is the only known place on Earth where nitrate has accumulated to the point that nitrate mining is commercially feasible.
While debate continues about the source of nitrate in the Atacama Desert, this is not relevant to the question of whether nitrate will accumulate in the absence of denitrification. It clearly does. It is, however, worth noting that measurements of oxygen isotope composition of this nitrate suggests that a significant proportion of it accumulated within the past 2,000,000 years as a result of atmospheric deposition resulting from photochemical fixation in the upper atmosphere.[52] Thus, in the absence of denitrification, nitrate appears to accumulate as a result of abiotic processes. As mentioned previously, low levels of atmospheric nitrogen on Mars may be attributable in part to accumulation of nitrates in the Martian regolith, where a biological nitrogen cycle is not thought to exist.
A Darwinian scenario may be conceivable for this step in the nitrogen cycle if certain assumptions are made. These include the existence of aerobic bacteria — a mechanism for accumulation of nitrate — and niches, like soils from which oxygen is occasionally excluded. In this scenario, some aerobic bacteria might have a weak ability to use nitrate instead of oxygen as an electron acceptor during respiration. Perhaps this could have been related to their ability to utilize nitrate as a nitrogen source and then reduce it to ammonia for amino acid production. Natural selection working on these bacteria, as they survived periods of oxygen starvation better than those that are completely dependant on oxygen, may ultimately have produced the denitrifying bacteria living today.
This scenario presents a number of problems. The first is the obvious appeal to unknowns. Were there bacteria in the past capable of utilizing nitrate as an electron acceptor during anaerobic respiration before there was a fully developed nitrogen cycle? No evidence supports this, and there is a commensurate lack of evidence for nitrate having accumulated significantly in the environment. The way in which organisms both assimilate nitrates (which will be discussed in the next section), and engage in nitrate respiration also suggests no linkage between the two processes. In these organisms, two significantly different nitrate reductases are produced.[53] For example, in E. coli, the respiratory enzyme is particulate and sensitive to oxygen while the assimilatory enzyme is soluble and the two enzymes are induced and repressed by different substrates. Evidently the processes of nitrate respiration and nitrate assimilation are biochemically distinct, and do not exhibit the kind of convergence needed to support the theory that they share a related evolutionary history.
Evolving nitrogen-reducing systems in a reducing environment appears to be out of the question, given the lack of oxidized nitrogen in such environments. In an oxidizing environment, even in the absence of biological fixation or nitrification, nitrates are likely to be present. In fact, they would presumably be the sole source of nitrogen for organisms lacking the ability to perform steps other than assimilation and amination in the nitrogen cycle. Assuming this to be the case, the ultimate problem of recycling nitrogen to the atmosphere might be temporarily suppressed by accumulation of nitrogen in living organisms and their byproducts, but this does not negate the ultimate need to recycle nitrogen to the atmosphere, and may even exacerbate it once nitrogen as either ammonia or nitrate reached excessive levels. The question then becomes, does this biological sink provide sufficient time for the stepwise evolution of other components of the nitrogen cycle? Ultimately, denitrification appears to be an indispensable part of the nitrogen cycle and unlikely to have evolved in Darwinian fashion independent of the rest of the cycle.
4A. ASSIMILATION[54]
Nitrate serves as a major crossroads in the nitrogen cycle. As already discussed, nitrate is produced via biological nitrification and abiotic atmospheric nitrogen fixation. Once it is in the form of nitrate, nitrogen can either be returned to the atmosphere as N2 during denitrification, or it can be assimilated by plants and bacteria. While nitrate is readily absorbed by plants and bacteria, it is only as ammonia that it can be utilized. The process of nitrogen assimilation involves conversion of nitrate to ammonia and the incorporation of that ammonia into amino acids.
Nitrates enter plant cells via a “proton-nitrate symport.”[55] Once in plant cells, nitrates are converted to nitrites by the enzyme, nitrate reductase. Highly toxic nitrite, a metabolite in the process, is rapidly sequestered in chloroplasts, thus protecting plants from harm. Inside plastids, nitrite is quickly converted to ammonia by another enzyme, nitrite reductase. Significantly, in at least some plants, the reducing power is provided by photorespiration which is dependent on the presence of oxygen.[56] In most organisms, assimilation is repressed by the presence of ammonia and induced by nitrate or nitrite.[57]
Microbial assimilation of ammonia to produce amino acids occurs first through the synthesis of glutamate, alanine, or aspartate.[58] These then serve as nitrogen donors via transaminases to form other amino acids. Ammonia, for example, may be used to aminate glutamate to produce the amino acid glutamine, by means of the enzyme, glutamine synthetase (GS) plus ATP. GS is the principle means by which ammonia enters the metabolic processes of plants. Then, by means of a glutamate synthase, known as GOGAT (Glutamine 2-OxoGlurate AminoTransferase), one out of two glutamines produced is converted back to glutamate to pick up yet another ammonium molecule. Each turn of the GS-GOGAT cycle results in a profit of one glutamine. From glutamine, nitrogen is passed on by means of transaminases to other molecules to form different amino acids. The process can also go in reverse. Ammonia assimilation occurs in both roots and leaves via this method.[59] Eventually, assimilated nitrogen is used to produce nucleotides and nucleic acids.
Assimilation is too complex to be considered in detail here. However, the importance of enzymes in transferring nitrogen to various molecules cannot be overstated. Note that nitrogen assimilatory enzymes contain nitrogen, the very element whose assimilation they facilitate. These processes are intimately tied to the actions of genes (whose nucleotides also contain nitrogen) which determine the structure of proteins. The actions of these genes are facilitated by several of the very enzymes, which they have, in fact, encoded. It is difficult to avoid the necessity of all of these entities being simultaneously present in order for the whole system to function.
4B. IS ASSIMILATION INDISPENSABLE AND UNBRIDGEABLE?
It has been generally thought that plants only take up nitrogen as ammonium or nitrate, but evidence is mounting that plants may also take in partially decomposed organic nitrogen in the form of amino acids, and possibly even more complex nitrogen-containing compounds.[60] Some evidence suggests that plants may access organic nitrogen by means of mycorrhizae. Given that the highest proportion of soil nitrogen is organic, organic nitrogen absorption should not be surprising.
Could assimilation be bridged by absorption of amino acids or other nitrogen-containing organic molecules? On the surface such an idea looks plausible, and it is not surprising that scenarios have been built around this idea as a way to entirely bridge the nitrogen cycle. However, on closer examination, simply bridging assimilation and nitrogen fixation by appealing to a reducing atmosphere in which amino acids, nitrogenous bases and other nitrogen-containing molecules are freely available creates its own set of problems.
The first and most obvious problem is that evidence favoring such a reducing atmosphere in the distant past is absent, and that the existence of such an atmosphere might have existed seems incredible. However, the purpose of this paper is not to argue against a reducing atmosphere; as already mentioned, these arguments have been convincingly made elsewhere.[61]
A second issue arises from the assumption that nitrogen-containing organic molecules could cross primitive cell membranes. This presents a significant issue as presumably more than one or two simple molecular pumps would be needed to transport any freely-available nitrogen-containing molecules. Pumps would be necessary as, even given some sort of primordial soup, the concentrations of amino acids and other nitrogen-containing molecules would be expected to be higher inside cells than outside.
Energy for pumping an array of nitrogen-containing molecules across primitive cell membranes would presumably not be available from photosynthesis as this requires the presence of the very amino acids that need to be pumped. Chemosynthesis, if it was hypothesized to have evolved before photosynthesis, would suffer from the same difficulty. It is not clear how any realistic energy source would circumvent this problem. In addition, proteins from which the pumps would be made are composed of amino acids. A scenario of this sort presents another chicken-or-egg dilemma. Organic membranes across which amino acids freely flow from areas of lower concentration to areas of higher concentration are unknown; membranes lacking protein pumps that concentrate amino acids on one side seem impossible. In addition, powering pumps is typically tied in some way to the use of nitrogen-containing nucleotides like ATP, which serve as the currency of energy metabolism within cells.
Accumulation of ammonia within cells presents a third issue. Energy to drive any kind of metabolism comes from the catabolism of molecules and ultimately from photosynthesis, or, less commonly, from chemosynthesis. In modern organisms some portion of this energy is derived from catabolism of nitrogen-containing molecules. How the waste nitrogen is handled will be dealt with in the next section. If a system for pumping amino acids across cell membranes existed in primitive cells, it would require energy from some source. If that source happened to be the amino acids themselves, then a mechanism would be required to be simultaneously in place to deal with the waste ammonia. This ammonia could not be consumed as a source of ammonia for amination, as these organic molecules would not yet be available without further complex protein-dependent biochemical pathways. In any case, there seems to be little reason for cells to make amino acids if they were freely available. Presumably waste ammonia would have to be pumped or diffuse out of the cells via some sort of protein channel. This presumes that a mechanism for getting energy from reduced organic molecules could serve as a source of energy in a reducing environment via either anaerobic respiration or fermentation.
Within certain biomes, for example boreal forests, organic nitrogen is cycled rapidly through ammonia which is absorbed directly by plants. In the absence of denitrification, organic material accumulates and is ultimately recycled via fires or goes on to form peat. Taken as a whole, some areas in the biosphere can do this without upsetting the overall balance of the nitrogen cycle, but, as noted in the discussion of denitrification, on a global scale such a system appears to be catastrophic in the end.
Ultimately, easier ways of getting nitrogen into organic molecules inside cells other than assimilation seem improbable, although they would be necessary in a reducing environment. Given that the current atmosphere is an oxidizing one, and this seems to have been the case in the ascertainable past as well,[62] assimilation is clearly necessary under current conditions, and presumably historically as well.
5A. EXCRETION AND DECAY
Plants make use of nitrogen in an efficient manner and usually do not excrete it. Animals present a very different situation. To obtain amino acids they are unable to make, they must consume plants, or other animals that consume plants. In this way, they acquire excess nitrogen that must be excreted. Nitrogen is excreted in different forms by different animals. Fish excrete nitrogen as ammonia, which though highly toxic, is greatly diluted in surrounding water. Mammals excrete less toxic urea, which still requires significant quantities of water and energy; producing urea uses 4 ATPs per urea molecule. Birds and reptiles excrete uric acid as a solid, which does not waste water, but uses even more energy. Excess pyrimidines and purines from nucleic acids are also treated and excreted, pyrimidines as ammonia or urea, but purines must be converted first to uric acid and, in mammals, farther modified to allantoin for excretion. Because primates are unable to produce allantoin they are consequently subject to gout when they consume purines in excess.[63] Excretion not only rids animals of excess nitrogen, but also returns nitrogen to the nitrogen cycle.
Both plants and animals die and leave remains that require recycling. Even while alive, they shed tissues; plants shed leaves and bark, for example, while animals shed skin cells and hair. Additionally, when animals eat other organisms, not all parts are consumed or assimilated. The excess, either the uneaten parts or the eaten unassimilated parts expelled as fecal waste, must still be recycled including any excreted waste nitrogen. All plant and animal parts ultimately undergo decay in which they are broken down to simpler molecules. Decay is facilitated by numerous soil organisms: algae and cyanobaceria on the surface, with many other varieties of organisms just beneath — bacteria, fungi, different kinds of worms, mites, many insects and even burrowing mammals, to mention only a few. Nitrogenous organic compounds, along with other organic molecules, are first broken down physically when they are consumed by a number of different soil animals. Nitrogenous wastes are eventually converted back to ammonia and thence into nitrites and nitrates (mineralization), and so on, in the continuing nitrogen cycle.
At any given moment, however, more than 90 percent of nitrogen in healthy natural soil is in the form of organic compounds — such as amino acids. In fact, a study in Alberta showed that only 2 to 5% of soil nitrogen is inorganic.[64] Soils with high organic content have higher overall nitrogen levels. A recent study of pristine riverine systems showed that an average of 80% of nitrogen in those waters was organic.[65]
The gradual breakdown of organic nitrogen maintains a long-term readily available and manageable nitrogen source that would eventually be lost to mineralization with subsequent loss to leaching should the breakdown occur too quickly, as may occur in moist tropical regions. Tropical forest plants quickly absorb available nutrients, so that those nutrients are usually tied up in living tissues. Widespread destruction of tropical forests leaves too few plants to quickly assimilate soil nutrients and very swift breakdown of organic molecules follows. Mineral nutrients — including nitrates and ammonia — rapidly leach from the soil, leaving behind only an insoluble and infertile hardpan.
Ultimately, nitrogenous waste products are all recycled, either back into the atmosphere or back into organisms via ammonia and nitrate, with nitrate serving as a major crossroads point in the nitrogen cycle. When the natural situation is perturbed by either overwhelming natural systems as when excessive amounts of nitrogen fertilizer are used, or by speeding up mineralization as when tropical forests are destroyed, the results are inevitably negative. A minimal set of organisms capable of performing each necessary step in the cycle must be present for a healthy functioning ecosystem. Even when this occurs it may still be difficult to reestablish a damaged ecosytem, as in the case of the hardpan left after rainforest degradation.
Establishing the nitrogen cycle in the first place would have been a remarkable feat no matter how it came about.
5B. ARE EXCRETION AND DECAY INDISPENSABLE AND UNBRIDGEABLE?
Animals require a mechanism to handle nitrogenous waste. That this is true is supported by the observation that no animal lacks a system to deal with these wastes, these systems are expensive to maintain and when they fail, animals die. Might it be possible to evolve a system in which nitrogen wastes were not recycled? The simple answer is no; logic precludes this. Nitrogenous wastes cannot be reasonably expected to accumulate forever without grinding nitrogen-containing life to a halt. This would be true for life composed only of simple bacteria, in an RNA world, in an oxidizing or in a reducing environment. Even with no actual waste production, whatever life or the precursors of life were, they cannot accumulate forever without exhausting all resources and creating stagnation.
In a Darwinian scheme, death is necessary to eliminate the less fit and components of dead organisms must be recycled for use by those that survive and produce more offspring. Dead or unfit organisms cannot accumulate forever, or Darwinian evolution would come to a halt. The necessary recycling of organic nitrogen is achieved by excretion and decay. Ultimately excretion and decay are indispensable, but are they unbridgeable?
Note that there is a difference between being bridgeable at the time life came into existence and in the present when there may be multiple redundant organic systems capable of doing the same or related tasks. For example, in theory it should be possible to drive biochemical pathways both forward and backward. Thus an anabolic pathway might hypothetically be adapted to work as a catabolic pathway if necessary or vice versa. While there are practical reasons why this does not happen, still, with no other options, this might be all that could be achieved. If this was the case, if an organism already had the ability to make certain kinds of polymers, then it might be able to use the same biochemical pathway to degrade those polymers. Whether the pathway worked anabolically or catabolically might depend on the circumstances in which it existed. The very same organism might make organic polymers under one set of circumstances and consume them under others. Over time different populations in different environments might be able to adapt their particular metabolism to the different tasks to which they put their biochemical systems.
The problem with this scenario is that it presupposes an anabolic pathway in the first place. It could not start out as a catabolic pathway as, without an abundance of polymers to breakdown, it would not be useful to have one. If an anabolic pathway already existed, would there be sufficient time to adapt it to do catabolic work — or for a separate catabolic system to evolve — before metabolite buildup overwhelmed the pathway, causing it to grind to a halt? Getting a precise answer to this question seems unlikely, but it is not necessary, as to get to this point involves a miracle in the first place — an anabolic pathway that is adaptable to a catabolic pathway in small steps that are all adaptive. Still, of all the various steps in the nitrogen cycle, this one may be the most amenable to being bridged in some way, either by adapting a biochemical pathway or by simply relying on occasional fires to return nitrogen to the atmosphere or soil as nitrogen oxides.
The process of excretion is not as easily bridged and seems to require mechanisms for the detoxification of nitrogen-containing waste products and their removal from cells and/or bodies. This is no trivial matter, whatever the waste product. Even in those animals that directly excrete ammonia, it requires specific protein channels or pumps and thus is not an easily bridgeable step.
DISCUSSION
Much of the argument made in this paper hinges on high reaction rates resulting from biological enzymes acting as catalysts and on the way in which biological systems drive reactions in specific directions. In theory, every component of the set of chemical reactions we call the nitrogen cycle occurs without biological intervention. But do these abiotic reactions occur at rates sufficient to maintain a cycle essential to life like the N-cycle? And are the reactions shifted in the necessary directions to make them work without accumulation of intermediate products at specific points in the cycle? With the possible exception of photolysis of ammonia, which is not a major component of the cycle, this does not appear to be the case. Obviously, if an abiotic nitrogen cycle existed that could sustain life; there would be no real need for a biological nitrogen cycle, just as there is no need for a biological water cycle.
A detailed critique of current Darwinian theories about nitrogen cycle evolution has not been undertaken. A careful search of the literature reveals many papers that mention evolution of the nitrogen cycle, but examination of them has not revealed a detailed model. Rather than discussing how the entire cycle could have evolved via some Darwinian mechanism, these papers generally discuss only the evolution and genetics of specific stages in the nitrogen cycle,[66] or they present what is imagined to be necessary assuming some Darwinian mechanism.[67] Inorganic nitrogen cycles have been proposed, but how they transitioned to the organic nitrogen cycle seen today is unclear, as is whether specific conditions under which these cycles are thought possible ever actually existed. In essence, the biological nitrogen cycle appears to present a naturalistic conundrum similar to Leslie Orgel’s observation about the citric acid cycle: “In my opinion, there is no basis in known chemistry for the belief that long sequences of reactions can organize spontaneously – and every reason to believe that they cannot. The problem of achieving sufficient specificity, whether in aqueous solution or on the surface of a mineral, is so severe that the chance of closing a cycle of reactions as complex as the reverse citric acid cycle, for example, is negligible.”[68]
If the general argument made in this paper is true — that the various steps in the nitrogen cycle all appear to be necessarily present within a limited temporal span, and do not appear to be the product of a Darwninan process — two possible evolutionary scenarios seem to be the best conjectures in the absence of some intelligent cause. The first is that the complete cycle evolved in a single organism and that, subsequently, this organism gave rise to the diverse groups of organisms that today participate in various steps of the nitrogen cycle. Over time some groups in this ancestral population may have lost various components and specialized in others. This scenario seems incredible given the profoundly different organisms involved. Lateral gene transfer might be invoked to explain away some of this problem, but in either case it requires evolution of the entire cycle in a single organism extremely early in the history of life. Given the complexity of the nitrogen cycle, such a scenario stretches credulity.
A second scenario would be to have various components of the nitrogen cycle evolve in different taxa which all happened to evolve them at around the same time or at least before accumulation of the products of one step accumulated to toxic levels. This would mean that, without any goal in mind, Darwinian mechanisms produced everything necessary for the cycle to work, while at the same time achieving enough coordination between the various steps in the N cycle to avoid the inevitable problems that logically follow from having at least some steps missing or out of balance with the others. This again seems incredible, given the finite window of time available, questionable selective pressure to produce all stages at once, and the need for a complete cycle to sustain the production of proteins in anything other than a hypothetical reducing atmosphere for which evidence is lacking.
Essentially this second scenario is similar to those explanations which invoke cooption in the production of irreducibly complex cellular machines and shares their speculative weaknesses. The difference is that an ecological system like the N cycle requires a number of organisms all evolving independently to achieve a fortuitous outcome necessary for their existence rather than a number of components serendipitously combining in a single organism.
It seems optimistic to suggest that organisms alone, without some intelligent guidance which cannot be provided by natural selection, could build a coordinated ecochemical cycle like the N cycle. Given the number of highly reactive nitrogen compounds possible, significant good fortune would be required to prevent evolution of biochemical pathways with products that preclude life. For example, why organisms would not evolve that produce cyanide (CN-) as a waste product is not obvious. Thus, there are significant constraints on how the N cycle could come into being.
It is also worth mentioning that both scenarios, evolution in a single organism or evolution in multiple organism, suffer from the problem that evolution of the biological nitrogen cycle could only take place in proteindependent organisms.
Reasons why powerful arguments to design in nature can be made based on biochemistry were outlined by Behe and may be summarized as:
- Biochemistry allow sex amination of the“rock-bottom level of life”[69].
- Chemistry and physics are sufficiently understood to allow evaluation of claims about the behavior of atoms
- Significant differences exist between what Darwinism claims atoms did, and what atoms are actually known to do.
In short, atoms are known to arrange themselves according to certain relationships we call physical laws. None of these laws or combinations of these laws is known to produce either machines like the protein machines found in living cells, or information like that found in the orderly arrangement of molecules in DNA.
In this paper we ask whether characteristics of design recognized by Behe in biochemical pathways and molecular machines inside individual organisms may be evident in “ecochemical” pathways where organisms interact in complex patterns of interdependence. The primary focus has been on whether a rigorous argument, equivalent to Irreducible Complexity (IC), as defined by Behe, can be made for the nitrogen cycle.
Should such a system be evident at the ecological level, it would be similar to IC, but would also exhibit significant differences from biochemical IC. For example, instead of macromolecules interacting within single organisms, these systems involve whole organisms and potentially communities of organisms interacting both with each other and with the inorganic constituents of the niche they occupy. We propose the term “Irreducible Interdependence” (II) to describe this kind of ecological system. To exhibit II, an ecological system must exhibit the following characteristics, which parallel and add to those outlined by Behe for IC systems:
- The system must not exhibit obvious plausible inorganic workarounds. In other words, potential gaps in the system cannot be reasonably bridged or bypassed by inorganic nature alone.
- It must exhibit a degree of specification indicating that there are not so many solutions to the problem that a solution is a probable product of chance.
- A given function or step in the system may be found in several different unrelated organisms. In the specific case of ecochemical pathways, it is not a specific organism that is irreducible, but the ability to perform a biochemical reaction necessary to the ecochemical pathway. The enzymes necessary to catalyze the reactions must exist somewhere in nature in sufficient quantities and be appropriately distributed to maintain the function of the system whether in one species or many species. Redundant species are not necessarily expendable as they may prove indispensable in forwarding the process under circumstances when other species have diminished abilities or are absent. Ultimately, it is not the species, per se, but the enzyme functions that count; “redundant” species constitute a vital buffer against perturbation of the system.
- While leaving the rest of the system intact, the removal of any one of some, but not necessarily all, individual biological steps must result in loss of function of the system. Note that this does not mean that every step must be essential to the system, but some must be. In addition, the steps that are removed must be too complex to have resulted from one or a very few serendipitous mutations. The more steps or components that are necessary for the system to function, the more compelling the argument that no direct path exists to build the system in a step-by-step manner via a series of relatively small opportune mutations, it does not mean that the possibility of building the system via some circuitous step-by-step process must be proven impossible, as eliminating an essentially infinite number of complex indirect imaginary paths would be unfeasible.[70]
- Individual steps in an II system may be adaptive for the individual species that carry them out and natural selection may be capable of acting on those individuals. Natural selection is not generally conceived as a process that can work on a global scale to construct a cycle like the N cycle in a teleological way. Because individual steps in an II system may be adaptive for individual species that evolve them (although the steps themselves may be IC) this constitutes a major difference between II and IC systems.
In the environment, II systems act in ways similar to biochemical systems within organisms, but they are different in that perturbations of biochemical systems typically results in reduced fitness or death of individual organisms, while disruption of II systems may result in local or even global collapse of ecosystems. Because individual steps in II systems may be spread across multiple organisms, as in the case of nitrogen fixation, disruption of II systems, especially on a global scale, may be more difficult than the relatively simple IC systems found in individual organisms. The natural redundancy built into II systems, along with their temporal and spatial distribution, may make their empirical study more challenging than biochemical systems within individual organisms.
Another difference between biochemical and ecochemical systems is that the sink for specific metabolites may be much larger in ecological systems than is possible within individual cells or organisms. For example, as mentioned in this paper, ammonia is highly soluble in water, thus the oceans represent a large sink for ammonia. This might allow life to exist for some time in the absence of systems to recycle ammonia back into organisms or back into the atmosphere as is done by the nitrification and denitrification steps that convert ammonia back into dinitrogen. Ultimately the question arises, is there sufficient time and does unguided nature possess the capacity to produce a solution to problems caused by buildup of reaction products before they make life impossible? Given the vastly increased reaction rates produced by enzymes, the time available must be relatively short, at least by conventional geological standards. In addition, no amount of time causes chemical reactions to do anything other than go to equilibrium whether at a rapid or slow rate. The ability to drive reactions in specific directions seems to be the purview of clever chemists in complex laboratories and biochemical/ecochemical pathways in living things.
Whether they are II or not, the intimate interdependencies of ecochemical systems are worth noting. Mechanisms of carbon fixation, and particularly photosynthesis, are tightly dependent on nitrogen fixation, and nitrogen fixation is dependent on them as well. This different kind of interdependence on a grander scale is illustrated in Figure 2. At least as currently understood, photosynthesis and nitrogen fixation appear to be vital to life. While the N cycle can be isolated and studied independently, its relationship to other biological and geological processes cannot be ignored if one wishes to gain an appreciation of how the cycle works in nature. Ultimately this ecochemical cycle has an ecology of its own!
The nitrogen cycle appears to meet the criteria listed for an II system. However, caution is warranted in drawing hard conclusions about this ecochemical pathway. Much of the argument made in this paper depends on what appear to be logical inferences, but not all of these have been tested, and testing, if possible, should take place before grand claims are made.
For example, while it is obvious that steps in the cycle, like denitrification, can be overwhelmed by the use of chemical fertilizers, it is not necessarily obvious that this is what would happen in the absence of denitrifying bacteria. It seems reasonable to expect that eutrophication would result, but this has not been tested. Thus, it seems that model systems need to be developed. In this specific case, perhaps self-contained communities of bacteria, which lack denitrifying bacteria, could be tested to see what the actual results might be. This would help to determine whether removal of denitrifying bacteria really does result in an increase in nitrate followed by a burst of growth and ultimately death of the system.
Despite the attractive and apparent simplicity of an ecochemical pathway like the nitrogen cycle, when spread across multiple organisms in the natural environment they are never as simple as a single pathway in an individual organism. With this caveat in mind — which should serve as a motivator for further laboratory research — the nitrogen cycle does give the appearance of potentially being an II system. This has profound implications for the timing of the appearance of organisms. Unlike other systems which might appear in individual organisms as a result of intelligent causes, if the nitrogen cycle and/or other ecological systems ultimately prove to be II, they would require a much grander action on the part of any Intelligence involved because all organisms making up this cycle must have obtained the components of the system they contribute within the time constraints imposed by the ability of inorganic sources and sinks to supply substrates and absorb products in such a way that life remains possible. In other words, the complete system is most reasonably understood as one that came into existence within a relatively short span of time. II systems, if they exist, appear to preclude the neo-Darwinian mechanism and are best explained as the product of a purposeful plan that was the product of Intelligence.
ENDNOTES
[1] By “ecochemical” we mean a series of biochemical reactions resembling a biochemical pathway, but distributed across multiple species instead of within an individual organism. Each step in the pathway may be mediated by a single species or multiple species all of which can do that particular biochemical reaction.
[2] Behe MJ. 1996. Darwin’s Black Box: the Biochemical Challenge to Evolution. NY: Free Press.
[3] Ibid., p 39.
[4] Behe MJ. 1998. Intelligent Design Theory as a Tool for Analyzing Biochemical Systems. In: Dembski WA, editor. Mere Creation: Science, Faith & Intelligent Design, p 177-194. Downers Grove, IL: InterVarsity Press.
[5] Persson J, Näsholm T. 2001. Amino acid uptake: a widespread ability among boreal forest plants. Ecology Letters 4:434-438.
[6] Smith RL. 1996. Ecology and Field Ecology, 5th edition. NY: Harper Collins.
[7] James N, Galloway JN. 1998. The global nitrogen cycle: changes and consequences.Environmental Pollution 102:15-24.
[8] A teragram = 1012 grams.
[9] Rachmilevitch S, Cousins AB. Bloom AJ. 2004. Nitrate Assimilation in plant shoots depends on photorespiration. Proceedings of the National Academy of Sciences (USA) 101(31):11506-11510.
[10] Information on nitrogen oxidation states and chemistry is widely available. A useful summary is found in: Whitten KW, Gailey KD. General Chemistry. Philadelphia: Saunders College Publishing, p 658-666.
[11] See for example: (a) Ohmoto H. 1996. Evidence in pre-2.2 Ga paleosols for the early evolution of atmospheric oxygen and terrestrial biota. Geology 24:1135-1138; (b) Walton JC. 1976. The chemical composition of the Earth’s original atmosphere. Origins 3:66-84.
[12] This is still the common textbook scenario. See for example: Campbell NA, Reece JB, Mitchell LG. 1999. Biology. 5th Edition. NY: Benjamin Cummings, p 492.
[13] Wells J. 2000. Icons of Evolution: Science or Myth? Why Much of What We Teach About Evolution Is Wrong. Washington DC: Regnery Publishing, p 9-22.
[14] Enormous amounts of nitrogen are industrially fixed today and applied as fertilizer. Hence the term, “natural method.”
[15] Peters GA, Meeks J C. 1989. The Azolla-Anabaena Symbiosis: Basic Biology. Annual Review of Plant Physiology and Plant Molecular Biology 40:193-210.
[16] Other plant genera that form symbiotic relationships with nitrogen-fixing bacteria include Casaurina, Coriaria, Gunera, Myrica, and coniferous plants such as Cycas, Macrozamia, and Podocarpus.
[17] Guerinot ML, Patriquin DG. 1981. The association of N2-fixing bacteria with sea urchins. Marine Biology 62:197-207.
[18] (a) Potrikus CJ, Breznak JA. 1977. Nitrogen-fixing Enterobacter agglomerans isolated from guts of wood-eating termites. Applied and Environmental Microbiology 33:392399; (b) Eutick ML, O’Brien RW, Slaytor M. 1978. Bacteria from the Gut of Australian Termites. Applied and Environmental Microbiology 35:823-828.
[19] Capone DG, Zehr JP, Paerl HW, Bergman B, Carpenter EJ. 1997. Trichodesmium, a Globally Significant Marine Cyanobacterium. Science 276:1221-1229.
[20] Moat AG, Foster JW. 1988. Microbial Physiology, 2nd Edition. NY: John Wiley & Sons, p 258.
[21] Ibid.
[22] Ruvkun GB, Ausubel FM. 1980. Interspecies homology of nitrogenase genes. Proceedings
of the National Academy of Sciences (USA) 77:191-195.
[23] Moat and Foster, p 261.
[24] Hill S. 1988. How is nitrogenase regulated by oxygen? FEMS Microbiology Review 54:111–130.
[25] Moat & Foster, p 257.
[26] Beringer JE, Brewin N, Johnston AWB, Schulman HM, Hopwood DA. 1979. The Rhizobium-Legume Symbiosis. Proceedings of the Royal Society of London. Series B. 204:219-233.
[27] Delves AC, Mathews A, Day DA, Carter AS, Carroll BJ, Gresshoff PM. 1986. Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiology 82:588-590.
[28] Schulze J. 2003. Source-sink manipulations suggest an N-feedback mechanism for the drop in N2 fixation during pod-filling in pea and broad bean. Journal of Plant Physiology 160:531-537.
[29] Voet D, Voet JG. 1990. Biochemistry. NY: John Wiley & Sons, p 726.
[30] Moat & Foster, p 252.
[31] Hubbel DH, Kidder G. 2003. Biological Nitrogen Fixation. University of Florida IFAS Extension SL16, p 3.
[32] Moat & Foster, p 254.
[33] Voet & Voet, p 726.
[34] Sprent JI. 1987. Cambridge Studies in Ecology: the ecology of the nitrogen cycle. Cambridge: Cambridge University Press, p 11.
[35] Moat & Foster, p 254.
[36] Voet & Voet, p 727.
[37] Warembourg FR, Roumet C. 1989. Why and how to estimate the cost of symbiotic N2 fixation? A progressive approach based on the use of 14C and 15N isotopes. Plant and Soil 115:167-177.
[38] Miller CD. 1989. Potential Hazards from Future Volcanic Eruptions in California. U.S. Geological Survey Bulletin 1847.
[39] Nye PH. 1981. Changes of pH across the rhizosphere induced by roots. Plant and Soil 61:7-26.
[40] (a) Robertson GP. 1982. Nitrification in forested ecosystems. Philosophical Transactions of the Royal Society of London B 296:445-457; (b) De Boer W, Kowalchuk GA. 2001. Nitrifcation in acid soils: micro-organisms and mechanisms. Soil Biology & Biochemistry 33:853-866.
[41] Postgate J. 1998. Genetics and Evolution. In: Postgate J. Nitrogen Fixation, 3rd ed., p 79-99. Cambridge: Cambridge University Press.
[42] Lang E, Jagnow G. 1986. Fungi of a forest soil nitrifying at low pH values. FEMS Microbiology Letters 38(5):257.
[43] According to the entry on “Air” (p 55) in Van Nostrand’s Scientific Encyclopedia, 6th ed. (Considine DM, editor. 1983. NY: Van Nostrand Reinhold Company), air is 75.54 % nitrogen by weight and the total mass of the atmosphere is 5.1 × 1021g giving a total mass of atmospheric N2 of 0.7554 ×5.1 x 1021g = 3.85 × 1021g. Note that this does not include dissolved nitrogen in the oceans and that a considerably smaller estimate, 4 × 1018g, is given in: Weast RC, editor. 1984. CRC Handbook of Chemistry and Physics, 64th ed. Baton Raton, FL: CRC Press, p B-23. Note that Galloway et al. (Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ. 2003. The Nitrogen Cascade. BioScience 53:341-356) seem to indicate a slightly higher number stating that the earth including its atmosphere contains 4 × 1021g of nitrogen of which 99 % is atmospheric N2. The reference they give is Mackenzie FT. 1998. Our Changing Planet: An Introduction to Earth System Science and Global Environmental Change. 2nd ed. Upper Saddle River, NJ: Prentice-Hall.
[44] Vitousek PM, Aber J, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman GD. 1997. Human Alteration of the Global Nitrogen Cycle: Causes and Consequences. Issues in Ecology Human Alteration of the Global Nitrogen Cycle
[45] Knowles R. 1982. Denitrification. Microbiological Reviews 46:43-70.
[46] Mancinelli RL, Banin A. 2003. Where is the nitrogen on Mars? International Journalof Astrobiology 2(3):217-225.
[47] Vitousek PM, Howarth RW. 1991. Nitrogen limitation on land and in the sea: How canit occur? Biogeochemistry 13:87-115.
[48] Korom SF. 1992. Natural Denitrification in the Saturated Zone: A Review.Water Resources Research 28:1657-1668.
[49] Galloway (see Note 43).
[50] (1 year/3 × 1012g)(3.85 × 1021g) = 1.3 × 109years, (1 year/5 × 1012g)(3.85 × 1021g) = 0.77 × 109years.
[51] Ericksen GE. 1983. The Chilean nitrate deposits. American Scientist 71:366-374.
[52] Michalski G, Böhlke JK, Thiemens M. 2004. Long term atmospheric depositions as the source of nitrate and other salts in the Atacama Deser, Chile: new evidence from mass-independent oxygen isotopic compositions. Geochimica et Cosmochimica Acta 68:4023-4038.
[53] Moat & Foster, p 263-265.
[54] For a recent overview of nitrogen assimilation, see: Oaks A. 1992. A Re-Evaluation of Nitrogen Assimilation in Roots. BioScience 42:103-111.
[55] In this mechanism, protons and nitrate are co-transported across root membranes with the high concentration of protons outside the root driving the “reaction.”
[56] Rachmilevitch S, Cousins AB. Bloom AJ. 2004. Nitrate assimilation in plant shoots depends on photorespiration. Proceedings of the National Academy of Sciences (USA) 101(31):11506-11510.
[57] Moat & Foster, p 263, 265.
[58] Moat & Foster, p 267.
[59] Lam H-M, Coschigano KT, Oliveira IC, Melo-Oliveira R, Coruzzi GM. 1996. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 47:569-93.
[60] One such recent study that points in the direction of organic nitrogen uptake is: Nasholm T, Huss-Danell K, Hogberg P. 2000. Uptake of Organic Nitrogen in the Field by Four Agriculturally Important Plant Species. Ecology 81:1155-1161. See also Sprent, p 19 (Note 34).
[61] Numerous citations could be given. For examples see Ohmoto and Walton (Note 11).
[62] Dutkiewicz A, Volk H, Georgwe SC, Ridley J, Buick R. 2006. Biomarkers from huronian oil-bearing fluid inclusions: an uncontaminated record of life before the great oxidation event. Geology 34:437-440.
[63] Masseoud D, Rott K, Liu-Bryan R, Agudelo C. 2005. Overview of hyperuricaemia and gout. Current Pharmaceutical Design 11:4117-4124. See also: http://steve.gb.com/ science/nitrogen_metabolism.html.
[64] AESA Soil Quality Benchmark Sites. Alberta Environmentally Sustainable Agriculture (AESA) Alberta Agriculture, Food and Rural Development. Available on the internet: http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/aesa1861?opendocument.
[65] Perakis SS, Hedin LO. 2002. Nitrogen loss from unpolluted South American forests mainly via dissolved organic compounds. Nature 415:416-419. See also: http:// www.nature.com/news/2002/020121/pf/020121-10_pf.html.
[66] Postgate, p 77-99 (Note 41).
[67] (a) Sprent, p 29-48 (Note 34); (b) Postgate JR, Eady RR. 1988. The evolution of biological nitrogen fixation. In: Bothe H, de Bruijn FJ, Newton WE, editors. Nitrogen Fixation: Hundred Years After, p 31-40. Stuttgart: Gustave Fischer.
[68] Orgel L. 1998. The origin of life a review of facts and speculations. Trends in Biochemical Sciences 23:491-495.
[69] Behe MJ. 1996. Darwin’s Black Box: The Biochemical Challenge to Evolution. NY: Free Press, p 15.
[70] As Behe points out (Ibid., p 40), “As the complexity of an interacting system increases, though, the likelihood of such an indirect route drops precipitously.”