©Copyright 2018 GEOSCIENCE RESEARCH INSTITUTE
11060 Campus Street • Loma Linda, California 92350 • 909-558-4548

“You are dust, and to dust you shall return.” This verse from Genesis 3 captures very well the fate of beautifully designed organisms after the entrance of sin into the world. But how long does it take for the organic molecules we are made of to break down after death? In general, the longer the time from death, the larger the amount of decay that should be observed. This is particularly true for soft tissue, the parts of an organism that are not mineralized (such as skin, muscles, or blood vessels). In 1993, Mary Schweitzer, then affiliated with the Museum of the Rockies, shared data suggesting the possibility of soft tissue and biomolecules preservation in a bone of Tyrannosaurus rex supposedly 68 Ma old [1]. Her findings were met with great resistance and skepticism. Similar observations of blood vessels, collagen, and osteocytes from dinosaur bone had been published by Roman Pawlicki and his colleagues since 1966 [2], but had not stirred much debate, probably because Jurassic Park, which popularized the subject, had not been written and filmed yet.
In the last two decades, Mary Schweitzer and her group found additional examples and used a widening array of analytical techniques to document their findings [3-9]. Consequently, the possibility of preservation of original dinosaur soft tissue and biomolecules is becoming more accepted, and this blog post reviews some of what has been published on the subject in the last year and a half.
Dinosaur specimens with soft tissue preservation reported in 2016-2017
Three different dinosaur skeletons, with bones in articulation or association, were described in the literature with special mention of or an emphasis on the presence of soft tissue.
The first, a ceratopsian ornithischian (Psittacosaurus sp.) from the Lower Cretaceous of China (Fig. 1), has skin preserved as a compressed film with characteristic pigmentation patterns [10]. The pigments are thought to represent original organic matter, more specifically melanin residues. This interpretation was based on SEM microscopy, showing ovoid impressions similar to melanosomes (melanin-bearing organelles).

The second, an ankylosaurine dinosaur (Zuul crurivastator) from the Upper Cretaceous of Montana, preserves integumentary structures such as osteoderms, with dark sheaths probably consisting of original keratin [11]. The paper describing the fossil does not present a chemical or microscopic analysis of the soft tissue, but mentions it as the subject for further future investigation.
The third dinosaur skeleton is also of an ankylosaur (Borealopelta markmitchelli), from the Lower Cretaceous of Alberta [12]. This articulated skeleton was found in marine deposits, in a formation where ichthyosaurs and plesiosaurs had been recovered but never a dinosaur. The encasing sediments show evidence of rapid burial of the carcass, with absence of scavenging in spite of some burrows in the surrounding deposits. The exceptional preservation of the fossil encompasses the molecular level, with remnants of organic matter in scales and horn sheaths of the body armor. Chemical analysis through mass spectroscopy indicated the presence of melanin in the organic residue, especially pheomelanin (a reddish-brown pigment). Melanosomes do not appear to have been preserved in this specimen.
Perhaps less impressive than soft tissue preservation, but equally interesting, is the evidence for preservation in dinosaur bone tissue of original molecular components that have a distinct chemical composition. This chemical signature was used to substantiate the presence of medullary bone (a type of bone produced by mature female birds during ovulation) in T. rex remains from the Upper Cretaceous of Montana [13].
Mark Armitage provided new documentation [14] of the exquisite preservation of blood vessels, osteocytes, and collagen at the submicron level in the Triceratops horridus horn and rib bones he discovered in Upper Cretaceous deposits of Montana [15]. The observations are based on microscopy and mostly from dissolved bone material. The next step in this project should be chemical analysis of the material. This example of soft tissue preservation is particularly stunning, given the relatively strong weathering of the horn (roots, fungal hyphae, and insect remains were found traversing the horn).
Mary Schweitzer and colleagues published a new study [16] on the remains of the hadrosaur Brachylophosaurus canadensis (Upper Cretaceous of Montana) that had previously yielded evidence for preservation of endogenous biomolecules [6]. Using a more rigorous protocol for sample preparation and higher resolution mass spectrometry techniques, they recovered 8 peptide sequences of collagen from the hadrosaur bone. Two of the sequences identified replicated some found in the previous study, whereas the other six were new.
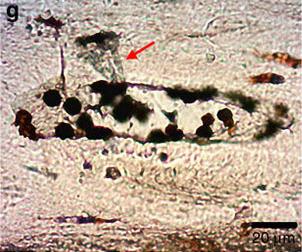
An important study presented results obtained on a sauropodomorph dinosaur (Lufengosaurus) from the Lower Jurassic of China [17]. Flat, transparent fragments of soft tissue located along and inside vascular canals in a rib bone (Fig. 2) were analyzed with infrared spectroscopy directly applied in situ and not on processed samples of bone. This non-destructive technique prevents the possibility of sample contamination during dissolution. The absorption spectrum observed was distinctive and typical of collagen. Moreover, particles of hematite (an iron oxide) were found in the vascular canals (Fig. 2) and lacunae left by osteocytes. The particles were interpreted as forming from iron ions attached to blood cells and iron-binding proteins. In the words of the authors, this study provided “undeniable, clear evidence that collagen and protein remains were preserved inside the osteonal central vascular canals of this early dinosaur.”
Finally, an intriguing abstract was presented at the 2017 meeting of the Canadian Society of Vertebrate Paleontology [18]. Fossils from the Upper Cretaceous Dinosaur Park Formation of Alberta showed an unexpectedly high rate of soft tissue preservation. A collection of bone samples from 25 specimens (16 of which dinosaurs), representing different degree of articulation and preserved either in sandstone or in mudstone, were dissolved and searched for soft tissue preservation. Of the 22 samples that successfully dissolved, 20 (including the dinosaur specimens) tested positive for soft tissues. It appears that soft tissue preservation in the Dinosaur Park Formation might be more common than expected, irrespective of the type of embedding sediment or degree of articulation of a specimen.
Recent papers discussing the preservation process
Understanding the pathway through which organic molecules can be preserved for tens to hundreds of millions of years is a significant challenge for those who subscribe to a “deep time” chronology. Proteins, for example, are thought to significantly degrade in shorter time frames of a few tens of thousands of years [19]. Therefore, several studies are attempting to explore potential mechanisms that could result in exceptional preservation of soft tissue in dinosaur remains.
Some have suggested that perhaps the blood vessels and osteocyte-like structures in dinosaur bones do not represent original organic material but are mimics created by bacterial biofilms colonizing the cavities of the bone [20]. However, Schweitzer et al. [21] presented data from actualistic experiments with bacterial biofilms to discard this hypothesis as inadequate. Interestingly, in the preparation of bone samples for their experiments they observed that removal of organics from bone is not easy, even with harsh treatment including repeated cycles of extreme heat, bleach, and enzyme treatment. Their suggestion is that when encased in dense cortical bone, labile organics can persist longer.
In their paper on preserved collagen from a Lufengosaurus bone, Lee et al. found that collagen was preserved only in the vascular canals, not in the bone matrix [17]. Given that the interior of the vascular canals often contained hematite particles, the authors suggested the collagen was preserved because it remained trapped between hematite concretions inside the vessels and the surrounding carbonated apatite minerals in the bone matrix.
Finally, some are still questioning the reliability of the results published by Mary Schweitzer and her group. For example, Buckley et al. [22] demonstrated that all the published putative dinosaur peptide sequences from T. rex and B. canadensis are matched by sequences of collagen from ostrich bone. Their suggested implication is that cross-contamination of the dinosaur samples with ostrich material in the lab cannot be ruled out.
Conclusive considerations
The discussion surrounding the preservation of dinosaur soft tissue is a fascinating example of a paradigm shift in science. Although still met with certain resistance, the evidence for endogenous biomolecular material in fossils has led to a proliferation of new observations and an openness to search for data that were previously overlooked, just because they were considered beyond the realm of possibility. It is clear that this field has a great potential for growth, including a better systematization of the type of molecules that are more commonly preserved in the fossil record and of possible differential levels of degradation and decay observed in these biomolecules at different stratigraphic levels.
This area of research is very relevant for origins model, because it has implications for the discussion on a long vs short chronology of life on the earth. Is it realistic to think that these original tissues were indeed preserved for tens of millions of years? Are they rather evidence for a much shorter time elapsed since the death of a fossilized organism, in the order of thousands of years? My impression is that the answer to these questions will not depend much on the evidence itself. When it comes to origins and historical sciences, “silver bullets” or unassailable proof of a model tend to be elusive. Those committed to a long chronology will probably attempt to normalize something that was formerly considered exceptional, presenting numerous scenarios of how preservation through “deep time” could be possible. Perhaps, a positive outcome of these efforts will be a better understanding of biomolecular structure, thermodynamics, decay pathways, and interaction with the surrounding chemical environment. However, those who subscribe to a biblical chronology will also have the opportunity to point out possible inadequacies of postulated mechanisms of preservation through “deep time.” Moreover, if soft tissue preservation turns out to be more common than previously thought, instead of “exceptional,” this line of evidence would also fit well with a short chronology and flood model of origins, not necessarily “proving” but being certainly compatible with a biblical worldview. Indeed, the most exquisite and pristine examples of original soft tissue preservation will likely remain a challenging puzzle for those who assign them ages covering periods of time so immense to be even hard to conceptualize.
References
[1] Schweitzer, M.H., Biomolecule preservation in Tyrannosaurus rex. Journal of Vertebrate Paleontology, 1993. 13(Supplement to n. 3): p. 56A.
[2] Pawlicki, R., A. Korbel, and H. Kubiak, Cells, Collagen Fibrils and Vessels in Dinosaur Bone. Nature, 1966. 211(5049): p. 655-657.
[3] Schweitzer, M.H., et al., Soft-tissue vessels and cellular preservation in Tyrannosaurus rex. Science, 2005. 307(5717): p. 1952-1955.
[4] Asara, J.M., et al., Protein sequences from Mastodon and Tyrannosaurus rex revealed by mass spectrometry. Science, 2007. 316(5822): p. 280-285.
[5] Organ, C.L., et al., Molecular phylogenetics of Mastodon and Tyrannosaurus rex. Science, 2008. 320(5875): p. 499.
[6] Schweitzer, M.H., et al., Biomolecular characterization and protein sequences of the Campanian Hadrosaur B. canadensis. Science, 2009. 324(5927): p. 626-631.
[7] San Antonio, J.D., et al., Dinosaur Peptides Suggest Mechanisms of Protein Survival. PLoS One, 2011. 6(6): p. e20381.
[8] Schweitzer, M.H., et al., Molecular analyses of dinosaur osteocytes support the presence of endogenous molecules. Bone, 2013. 52(1): p. 414-423.
[9] Schweitzer, M.H., et al., A role for iron and oxygen chemistry in preserving soft tissues, cells and molecules from deep time. Proceedings of the Royal Society B: Biological Sciences, 2014. 281(1775).
[10] Vinther, J., et al., 3D Camouflage in an Ornithischian Dinosaur. Current Biology, 2016. 26(18): p. 2456-2462.
[11] Arbour, V.M. and D.C. Evans, A new ankylosaurine dinosaur from the Judith River Formation of Montana, USA, based on an exceptional skeleton with soft tissue preservation. Royal Society Open Science, 2017. 4(5): p. 161086.
[12] Brown, C.M., et al., An Exceptionally Preserved Three-Dimensional Armored Dinosaur Reveals Insights into Coloration and Cretaceous Predator-Prey Dynamics. Current Biology, 2017. 27(16): p. 2514-2521e3.
[13] Schweitzer, M.H., et al., Chemistry supports the identification of gender-specific reproductive tissue in Tyrannosaurus rex. 2016. 6: p. 23099.
[14] Armitage, M.H., Preservation of Triceratops horridus tissue cells from the Hell Creek Formation, MT. Microscopy Today, 2016. 24: p. 18-23.
[15] Armitage, M.H. and K.L. Anderson, Soft sheets of fibrillar bone from a fossil of the supraorbital horn of the dinosaur Triceratops horridus. Acta Histochemica, 2013. 115(6): p. 603-608.
[16] Schroeter, E.R., et al., Expansion for the Brachylophosaurus canadensis Collagen I Sequence and Additional Evidence of the Preservation of Cretaceous Protein. Journal of Proteome Research, 2017. 16(2): p. 920-932.
[17] Lee, Y.-C., et al., Evidence of preserved collagen in an Early Jurassic sauropodomorph dinosaur revealed by synchrotron FTIR microspectroscopy. Nature Communications, 2017. 8: p. 14220.
[18] van der Reest, A.J. and P.J. Currie, Preliminary results of an investigation into the preservation of soft tissue structures in bone from the Dinosaur Park Formation. Vertebrate Anatomy Morphology Palaeontology, 2017. 4: p. 49.
[19] Wadsworth, C. and M. Buckley, Proteome degradation in fossils: investigating the longevity of protein survival in ancient bone. Rapid Communications in Mass Spectrometry, 2014. 28: p. 605-615.
[20] Kaye, T.G., G. Gaugler, and Z. Sawlowicz, Dinosaurian soft tissues interpreted as bacterial biofilms. PLoS One, 2008. 3(7): p. e2808.
[21] Schweitzer, M.H., A.E. Moyer, and W. Zheng, Testing the Hypothesis of Biofilm as a Source for Soft Tissue and Cell-Like Structures Preserved in Dinosaur Bone. PLoS One, 2016. 11(2): p. e0150238.
[22] Buckley, M., et al., A fossil protein chimera; difficulties in discriminating dinosaur peptide sequences from modern cross-contamination. Proceedings of the Royal Society B: Biological Sciences, 2017. 284(1855).
Ronny Nalin, PhD,
Geoscience Research Institute