©Copyright 2018 GEOSCIENCE RESEARCH INSTITUTE
11060 Campus Street • Loma Linda, California 92350 • 909-558-4548

AN INTERVENTIONIST THEORY OF NATURAL SELECTION AND BIOLOGICAL CHANGE WITHIN LIMITS
by
Leonard R. Brand
Professor of Biology and Paleontology
Department of Natural Sciences
Loma Linda University
and
L. James Gibson
Geoscience Research Institute
WHAT THIS ARTICLE IS ABOUT
This paper proposes that mutation and natural selection can produce biological change, but are not sufficient to explain the origins of biodiversity and complexity. Instead, the authors argue that genetic complexity is the result of intelligent design, and was at a maximum when life on Earth first came into being. Mutation tends to produce variants of equivalent complexity at best, and more generally results in reduction of genetic complexity. Some genetic variants may be adaptive in particular environments, but the overall tendency of genetic change is toward genetic loss and degeneration. Natural selection acts to prevent, or at least slow down this process by eliminating individuals that are genetically inferior.
The rate of biological change may depend on environmental conditions, and would be especially rapid in the recovery phase of a worldwide catastrophe. Small, isolated populations and changing environmental conditions would combine to promote genetic change and speciation. This effect would be enhanced if genetic systems were designed to respond to environmental stress. Such responses could include an increase in mutation rates, environmentally triggered gene activation or deactivation, and changes in the timing of gene activity. Favorable gene combinations could then be favored by natural selection, in some cases resulting in the rapid appearance of new species.
Evolution theory has been highly successful in stimulating research and in explaining many biological phenomena. Is there a scientifically viable alternative to the naturalistic understanding of evolutionary genetics? We believe that there is. This article will compare the naturalistic theory of the origin of diversity with a theory of limited genetic change after the major groups of organisms were brought into being by informed intervention. This latter theory recognizes that nature follows predictable laws and that a scientist can count on these laws to be consistent, but does not deny the possibility of intelligent intervention in the process of origins, or of divine involvement in maintaining the constancy of the laws of nature.
This presentation is simply a progress report on our thinking on this subject, and does not claim to answer all of our questions. The theory will no doubt change as we gather more data.
In our theory the mechanisms for microevolution and speciation are, in many respects, not significantly different those in from currently accepted evolutionary theories, except for some basic points which will be discussed below. However, it has a different starting point and implies a very different history of life. In each section our understanding of the naturalistic theory will be briefly summarized, and an interventionist alternative will then be presented. To be fair to the authors of papers cited here, we wish to emphasize that most of them would not support the basic premise of this paper. We cite them only for specific ideas or data, and we believe that our reinterpretation is not inconsistent with the data cited. Some terms will be defined here: informed intervention is a general term referring to any divine involvement in history, including creation of life forms; microevolution is genetic change within a species; speciation is the development of new species; and megaevolution is evolutionary change which produces new families and higher taxonomic categories (Simpson 1953). We will not use the term macroevolution, because variation in the definition of that term limits its usefulness in this discussion. A common definition of macroevolution, as it is used in the scientific literature, is evolution above the species level (Ridley 1993).
PHILOSOPHICAL FRAMEWORK
A. Naturalistic evolution
It is assumed that every event, past or present, follows natural law. Science will accept only explanations of biological or geological events and processes that are based on the uninterrupted operation of natural laws, which are potentially understandable by science. Hypotheses that require or imply the existence of any type of divine intervention in earth history at any time are not acceptable.
B. Interventionism
On a day-to-day basis the processes of nature follow natural laws. Living organisms are like "machines" in the sense that we can figure out how they work and what laws govern their structure and function. Thus, scientists who subscribe to this paradigm can work and think much like naturalistic scientists, with one important exception: they do not a priori rule out the possibility that an intelligent superior being has, on some occasions, intervened in biological or geological history, particularly in connection with the origin of life forms. Such interventions could have involved the use of laws of nature that are well understood by God, but are beyond current human knowledge. Science cannot test these possible interventions, but science may recognize evidence that points to the existence of these discontinuities or unique events in history. This difference in approach is based on the conviction that if such discontinuities have occurred, it is better to recognize their existence than to ignore them.
ORIGIN AND DIRECTION OF EVOLUTIONARY CHANGE
A. Naturalistic evolution
According to naturalistic evolution theory, life on earth began with the evolution of the cell, from which developed the simplest forms of organisms. All structurally complex organisms evolved from these ancestors. All new genes or new information ultimately arose by mutation and recombination. Mutations occur randomly. Most are deleterious and will lower the individual's fitness or adaptation to its environment (Cain 1989, Maynard Smith 1989). New combinations of the genetic material are formed by reshuffling of combinations of characters during sexual reproduction. Natural selection eliminates the deleterious mutations and preserves the available combinations that are best adapted, in the organism's environment, for maximizing successful reproductive effort (Endler 1986).
Within each taxon (taxonomic group, such as a genus or family), the first forms did not have the advanced characteristics of that group, but had primarily the characteristics of the group that was its immediate ancestor. Within each taxon, evolution progressed from the ancestral state toward forms with more derived characteristics (new characters that were not present in their ancestors) in their external appearance, as well as in their anatomy, physiology, behavior, and ecological adaptations. At lower taxonomic levels (within a species, genus, or family) these derived characteristics would not necessarily be more complex, but at some level in the evolution process structures and physiological systems were evolving that did not exist before. The overall picture is of the evolution of the complex whole of modern life from structurally simple initial life forms.
B. Interventionism
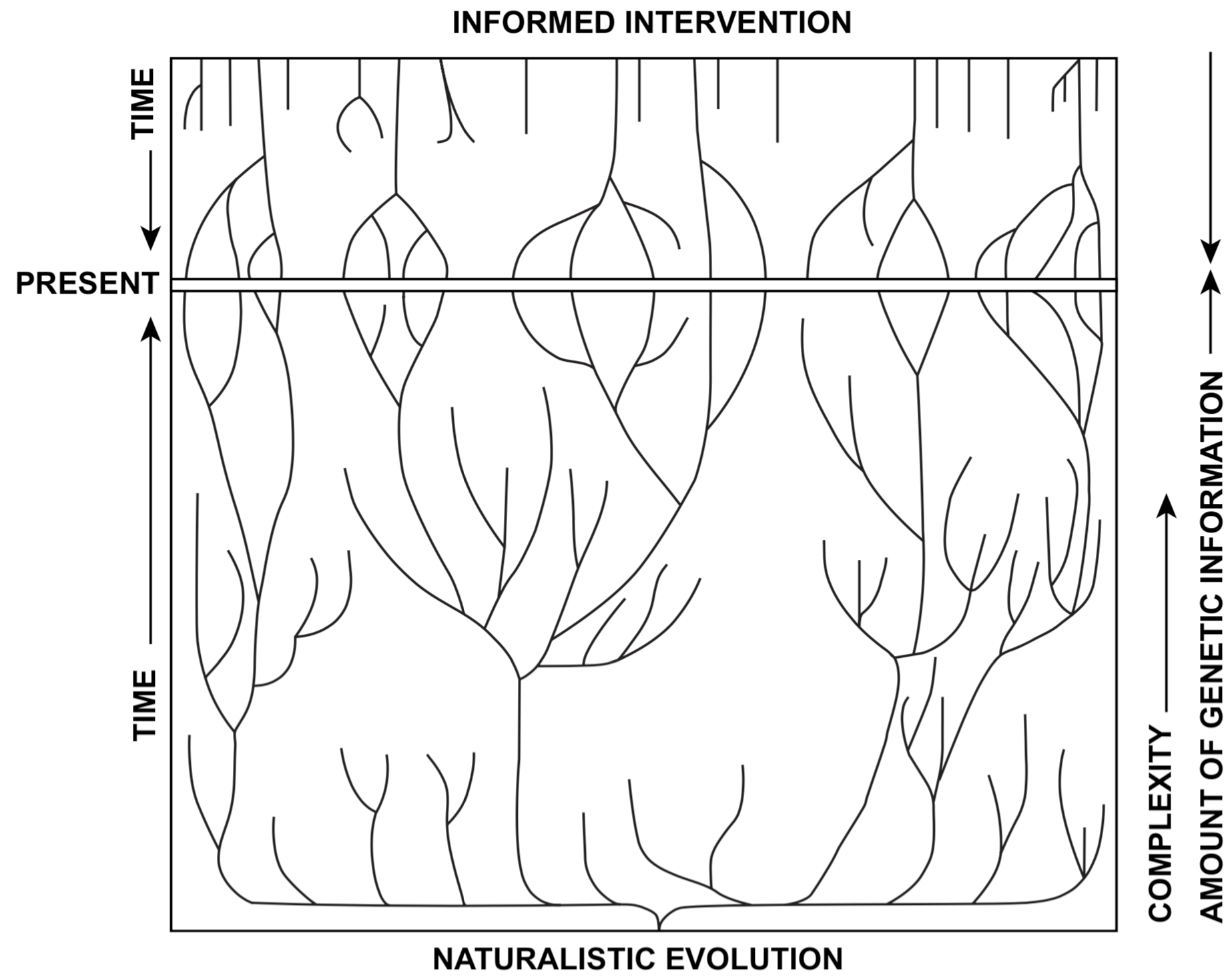
According to the interventionist theory, at the creation of life on earth, representatives of all major extant and extinct groups of plants and animals were present. Living things were as complex at the beginning as they have ever been. The earliest forms were at least as complex, although not necessarily as specialized, as any modern-day representatives of their group in their external appearance, anatomy, physiology, behavior, and ecological adaptations. In these early populations the amount of genetic information and the potential for genetic diversity per species may have been at the highest level that it has ever reached. The high point of the complexity of life on earth was at the very beginning.
Complexity in plants and animals was the result of intelligent design. Organisms were designed with a genetic system which possessed the capacity for genetic variability that would permit the organism to adapt physiologically to changing conditions and to produce new species and varieties that would be new variations on existing themes. At first this process did not involve the primarily destructive element of random mutations, but utilized the potential for variability built into the genetic system. The first populations of the original species were not all alike - there was considerable variation in their characteristics - and they probably had a genetic system capable of generating additional diversity when needed, by producing new alleles or by switching on stored, unexpressed genes.
As time went on, environmental changes occurred that increased the mutation rate. Radiation and other mechanisms began to produce random genetic damage (i.e., mutations), and/or there was a decrease in the efficiency of the gene replication and repair mechanism. Since mutations are mostly deleterious, the damage must be controlled to prevent life from going extinct. Natural selection has been the agent which has eliminated the less-fit individuals, and has assured that, on average, those which reproduce are the healthiest and best adapted to the environment in which they live.
Within each group of organisms, the origin of new morphological variation has involved two basic components. First is adaptation to changing conditions by production of new alleles for existing genes and selection for those alleles best suited to the environment, by the generally accepted processes of microevolution. An example of this type of adaptation to the environment is the development of dark pelage by a rodent living on dark soil (Dodson & Dodson 1985, p. 194). Another example is the behavioral adaptation of marmots to differences in climate (Barash 1974). Marmots have adapted to alpine areas with short summers and to milder, low-elevation climates by changes in aggressiveness, coloniality versus territory defense, and rate of maturation. They reach sexual maturity in two years in alpine areas, and in one summer in lowland areas. This adaptation process does not necessarily involve either increase or decrease in complexity, nor the evolution of new genes or structures. Perhaps it could involve the turning on and off of genes by environmental signals. Thus new characteristics might be caused by formerly inactive genes.
A second component of morphological variation is the tendency toward loss of genetic information in organisms since their origin. Examples are loss of flight by some birds and insects, and loss of sight by cave organisms. We argue that organisms today are, on the whole, less complex and less adaptable, and the interactions between organisms in ecosystems are less finely tuned, than at the beginning of life on earth. In most cases natural selection tends to slow down the loss of information by eliminating defective individuals, unless the environment allows or favors the genetic loss.
LOSS OF GENETIC INFORMATION
A. Naturalistic evolution
Since most mutations are harmful, there is the potential for effective loss of genetic information, unless natural selection is able to eliminate the damaging mutations. An animal species has a certain amount of genetic material, some of which is absolutely vital for survival of the species. Another portion of the genetic information is optional, and includes behavioral and physical traits that the species can lose and still be viable (Carson 1975) (Figure 1). Which features fall in this category will depend on the environment.
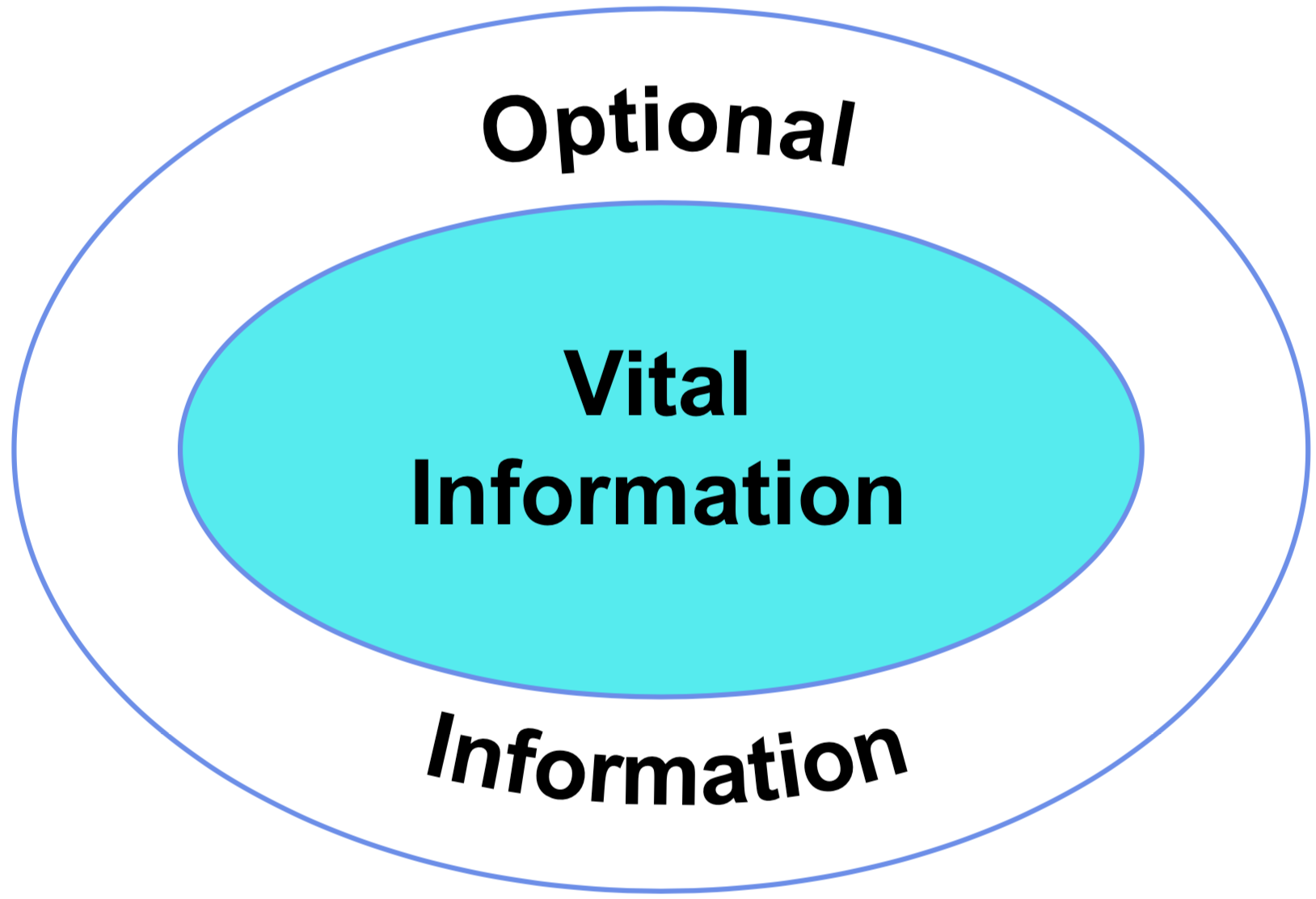
For most birds flight is vital, and loss of flight would probably doom the bird to extinction. However, on an island with no predators, losing the ability of flight might not be a problem and might even be an advantage in a tropical storm that can blow flying birds out to sea. There are a number of species of flightless birds, and most of these are on islands (Diamond 1981). Flight is optional in that situation, and this illustrates how a certain amount of genetic loss is possible. Other examples of genetic loss are blind cave salamanders and parasites that lack a digestive system.
Where have parasites such as tapeworms come from? It appears that their origin involves the loss of much genetic information as they degenerated from a free-living state. Tapeworms do not have some organs that similar, non-parasitic worms have. They don't have a digestive tract, but are essentially a highly developed reproductive system that lives in the intestine of their host. All the nourishment they need is absorbed through their skin. In this situation the tapeworm doesn't need a digestive tract; all it needs is a way to reproduce itself and maintain its location. If ancestral tapeworms with normal digestive tracts mutated, the loss of those digestive tracts would not have been disadvantageous, because of the nutrients available from the host. In this situation an organism can lose much more than is possible in other environments, and still be viable. These degenerate parasites exemplify change by loss of information.
B. Interventionism
Interventionist theory accepts the explanations given above for flightless birds, cave salamanders, and parasites. What would be the purpose of creating tapeworms and mosquitoes? It seems much more likely that these and other parasites have reached their present form through degeneration - loss of genetic information leading to dependence on parasitism. Cave salamanders and flightless birds have lost certain traits through mutation and natural selection, as described above, in the process of adapting to new environmental situations.
The interventionist theory presented here also proposes that loss of genetic information has not only been involved in the extreme cases described above, but has been a subtle and pervasive part of the genetic change in animals and plants since their original creation. The following example of possible loss of information is probably more typical than the type of loss experienced by some parasites or by blind salamanders. William Dilger studied the behavior of African lovebirds of the genus Agapornis, which are in the parrot family (Dilger 1960, 1962). He arranged the species of lovebirds in an evolutionary sequence. At one end of the sequence is a species that does not have the specialized features of some other lovebirds; it is very plain colored, has a simple courtship ritual, and makes a crude nest. The species at the other end of the Agapornis family tree consists of beautiful, colorful birds with more complex courtship, and which build elaborate covered nests. Several species are intermediate between them.
The usual interpretation of such a sequence is that the plain lovebird, with fewer characteristics unique to lovebirds, was near the beginning of the family tree, and the species with more specialized lovebird characteristics was the most highly evolved. But how can we be so sure that the changes didn't go the other direction? How would we decide? Usually the decision would be based on the initial assumption that these lovebirds have evolved from other, related types of birds. If that is done, it is reasonable to assume that the species with the least specialized lovebird characters is closest to the base of the lovebird evolutionary tree.
If we do not assume that all creatures have evolved progressively (in this instance, from another kind of bird), we can also consider the option that their evolution went the other way, starting with a lovebird with the most uniquely lovebird behavior and bright colors. Since the origin of those lovebirds, some species have lost varying amounts of genetic information, depending on the selection pressures to which each has been exposed. What has been lost are some of the specialized features the optional information that is not required to be a viable lovebird.
The result of the above-postulated process of genetic loss is that while the number of species of lovebirds has increased, there has still been a tendency toward loss of information. Many species are highly specialized and live only in a narrowly defined ecological niche. That is part of the reason why we have such a problem with extinction of species today. Man changes the environment, and many species cannot adapt to these changes because they have lost the ability to adapt. In contrast, some species are quite variable, or polymorphic, and adaptable. The coyote today is an adaptable species and has increased its numbers and its range, while less-adaptable species are becoming extinct.
Naturalistic evolution theory recognizes that groups of organisms may become divided into many species, each adapted to a specific niche. This specialization may be accompanied by loss of features or abilities that are needed by more generalist species. Hinegardner (1976) indicates that species with lower amounts of DNA tend to be more specialized. Our theory proposes a similar concept, except that the process started with a rich array of created life forms. Since the original creation of organisms, populations that were originally adaptable, with a high level of genetic information, have often become highly specialized, possibly with less genetic information per species. During this process many taxa have also divided into numerous species, with each species being specialized. Division of the original groups into the many specialized species of today is not just the latest minor episode in the history of life, but a major part of the change that has occurred since life began on this earth. Figure 2 illustrates the basic differences between the two theories.
NATURAL SELECTION
A. Naturalistic evolution
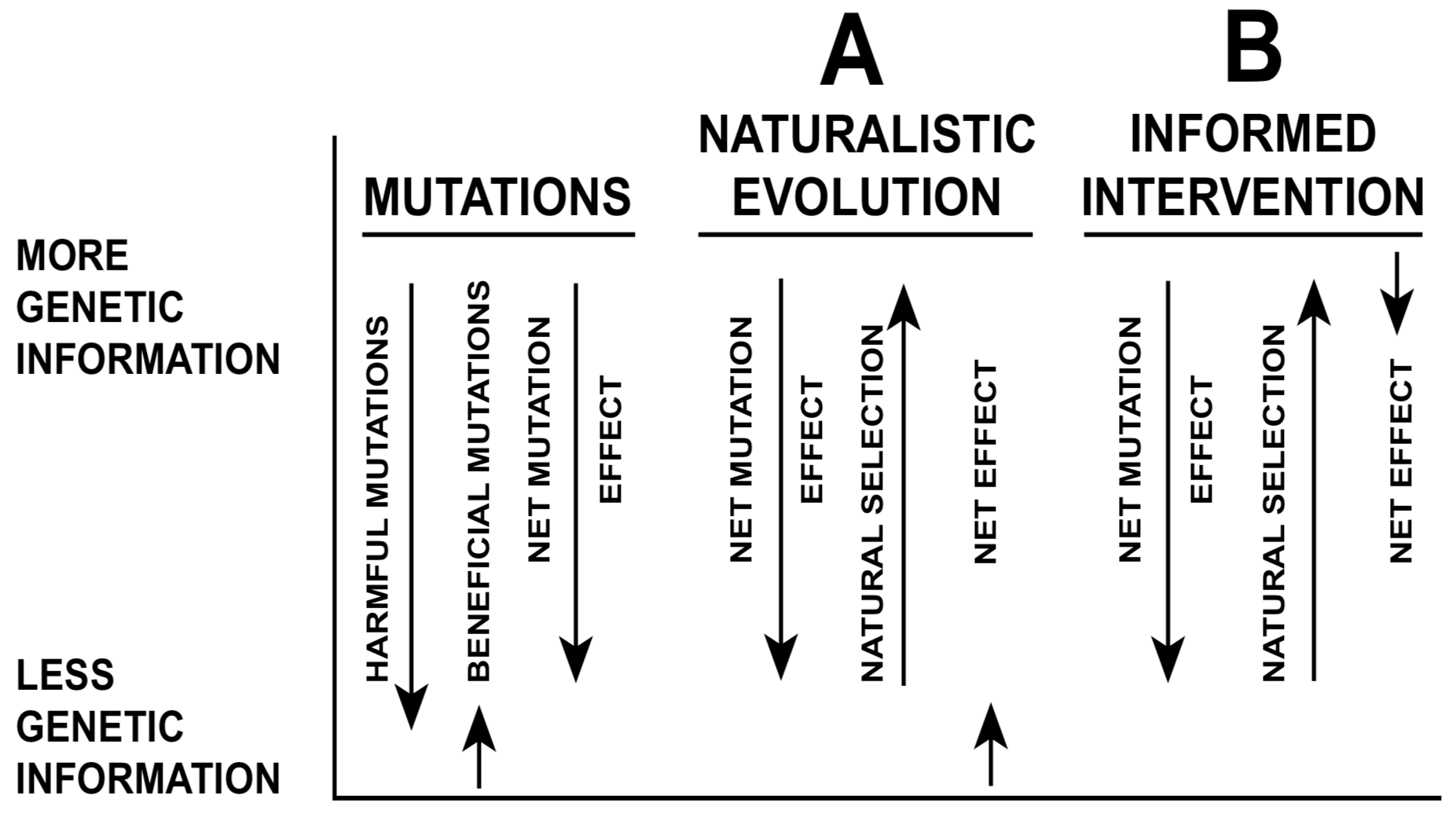
The naturalistic theory of evolutionary change begins with the genetic material provided by random mutation and recombination. Natural selection is the key process that rises above the randomness of mutation and selects the appropriate features to improve the adaptations of organisms. Most mutations are harmful, but natural selection is effective in eliminating most destructive mutations and preserving the beneficial ones, and consequently the net effect is upwards, towards improved adaptation to the environment, and ultimately the production of new genes, new adaptations, and even new organ systems (Figure 3A).
B. Interventionism
Both naturalistic evolution and informed intervention recognize natural selection as an important factor in the microevolution process, but the specific role of natural selection differs in the two theories. This interventionist theory recognizes the same forces, but suggests that the balance of forces is different. Edward Blyth anticipated Charles Darwin's theory of natural selection, but Blyth was not an evolutionist. He viewed natural selection as a conserving force, maintaining the species by eliminating the weak individuals (Eiseley 1979). Lester and Bohlin (1989) have suggested that Blyth was more correct than Darwin and that evolutionary change occurs only within limits. Informed interventionism suggests that mutation and natural selection are not able to produce an increase in complexity by generating new genes and organs. They are only able to change animals within the constraints of their original genetic potential, and to slow down the slide toward oblivion which would occur if the accumulation of harmful mutations were not held in check. Natural selection is nearly able to offset most of the deleterious effects of mutation, but the net evolutionary change is slightly downward (Figure 3B). Natural selection acts as a brake, to eliminate many of those individuals that have been weakened by mutations, and thus to slow down the destructive forces that can come from mutation.
This theory of natural selection is actually not a new or radical idea, and does not seem to go against the data that are available, even though Ridley (1993, p. 508) claims that "no one seriously doubts that the microevolutionary processes . . . [described earlier in his book] are fundamentally responsible for all evolution in the history of life." He does not support that claim with convincing genetic evidence that the proposed mechanism can accomplish the task, and some other non-interventionist scientists question whether natural selection can actually do some of the things that the neo-Darwinian synthesis maintains that it does (Arthur 1984, ch. 4; Bakker 1985; Ho & Saunders 1979; John & Miklos 1988, p. 336; Løvtrup 1987, ch. 12). They are not suggesting that animals were created, but that the traditional process of point mutation and natural selection is not the process that generates significant evolutionary change.
Interventionist theory recognizes that natural selection is a significant force, but suggests that it is not able to generate significant new structures, and that there is no other evolutionary mechanism that can do so.
EVOLUTION RATE
A. Naturalistic evolution
In naturalistic theory all new variability is ultimately the result of random mutations. Reshuffling of the genetic material provides many new combinations of traits for natural selection to act on, but the raw material is only provided by mutation. Mutations occur at random in relation to the needs of the organism, and most mutations are deleterious. Therefore evolutionary rates are usually very low; significant morphological change and megaevolution require a great amount of time.
B. Interventionism
Even though interventionists are often thought of as anti-evolutionists, the fact is that young-earth interventionists have to believe in a far more effective and rapid process of morphological change than non-interventionists. They have a shorter time period for the evolution of a large number of species and genera of organisms. Is that realistic? Actually there are important features of interventionist theory that would be favorable to rapid rates of change. First of all, the major taxa were in existence from the beginning. All that is needed is a process of diversification within each major taxon. The interventionist theory does not depend on new structural and biochemical traits evolving through mutation and natural selection. Change comes rather from a sorting out of genetic potential that was already present and from some loss of information and from differential gene expression. Net evolutionary change has been downward, or toward loss of information. Thus the evolution process has not been dependent on uncommon beneficial mutations, but utilizes the high level of genetic information that was a part of the original design. When the influence of the environment permits additional change by loss of information, the numerous deleterious mutations whose effects are otherwise held in check by natural selection speed the process of biological change. Thus expected rates of genetic change would be much higher than predicted by the naturalistic theory.
According to the theory presented here, much of our current taxonomic diversity has been the result of limited evolutionary change after a worldwide catastrophe. The original groups of plants and animals have diversified into multitudes of species, as they adapted to fill specific niches in the changed conditions after the catastrophe. If we consider the conditions that would likely exist after such a worldwide catastrophe and compare them with factors that are known to favor rapid genetic change, we find that conditions at that time would be ideally favorable for rapid change.
1) An abundance of potential, unoccupied niches to which organisms could adapt. Animals that have successfully colonized islands have often developed a large number of species. Examples of this are the fruit flies and honeycreepers of Hawaii, and the Darwin's Finches of the Galapagos Islands. Apparently this speciation is facilitated by open niches and the resulting lack of competition (Ford 1964, ch. 2).
2) Before the development of mature, balanced ecosystems, population dynamics would be unstable. This would result in flush/crash population dynamics: populations of animals expand, with all genotypes surviving, until they use up their food supply or until expanding predator populations catch up with them. The resulting population crashes produce the population bottlenecks (a time with few individuals in the population) favorable to speciation. Those individuals best adapted to particular niches will have the best chance of surviving the crash. Several, or many, species could be created simultaneously by a series of such cycles (Carson 1975; Mettler et al. 1988, p. 295).
3) Rapid geologic and environmental changes would favor the separation of organisms into isolated populations, which also facilitates speciation (Mayr 1970). This might have been particularly important for aquatic organisms, plants, and terrestrial invertebrates, which would likely have survived the global catastrophe in many scattered, isolated pockets. As the animals moved out over an empty world after the catastrophe, there would be almost limitless opportunities to occupy available new niches and speciate. In this situation, ecosystems initially would have been simple, and relatively unstable. Until mature ecosystems developed, many population fluctuations would likely occur. These, along with rapid geologic changes in the recovery period, would divide animal populations into smaller populations. The result would be a potential for very rapid rates of biological change after the global catastrophe (perhaps the most favorable situation for speciation we could imagine). The rate of change would slow down as environments and population dynamics stabilized, available niches were filled with increasingly specialized species, and ecosystems became more complex and balanced.
The overall implication of this theory is that evolution within the potential of the genetic system can be very rapid when conditions are favorable. Most of the modern (recent, or Holocene) species of animals evolved during the first few hundreds or thousands of years after the global catastrophe. Although it is commonly assumed that speciation takes hundreds of thousands or millions of years, even in modern times introductions of monkeys, birds, copepods, and moths to new geographic areas has produced change equivalent to new subspecies or species in time spans of 30 to 1,000 years (Ashton, Flinn & Griffiths 1979 [green monkeys]; Baker 1987 [mynas]; Johnson 1953 [copepod]; Johnston & Selander 1964 [house sparrows]; Zimmerman 1960 [moths]).
There is evidence that population bottlenecks usually reduce genetic variability (although usually only rare alleles are lost). This is a possible challenge for our theory of post-catastrophe evolution, because of the expected loss of genetic variability in those species with small numbers of individuals surviving the catastrophe. This leads us to suggest that there must be mechanisms to rapidly increase genetic variability after a population bottleneck. Observations of much higher genetic variability than expected after experimental or natural bottlenecks provide some evidence for the existence of such mechanisms (Carson & Wisotzkey 1989; Dessauer, Gee & Rogers 1992; Mettler, Gregg & Schaffer 1988, p. 296; Terzian & Biemont 1988). There is evidence that environmental or genetic stress produces genetic instability, with increased rate of recombination and increased mutation rates resulting from higher activity of movable elements (jumping genes) (Fontdevila 1992; Parsons 1987, 1988). Movable elements seem to produce most spontaneous mutations in Drosophila (fruit flies) (Langridge 1987) and in other eukaryotes (Reanna 1985). They have been implicated also in transferring genetic information from one type of organism to another, even from one kingdom to another (Amabile-Cuevas & Chicurel 1993). Some have even suggested that environmental stress can "induce" mutations which will be beneficial to the organism, although that is highly controversial (Cairns, Overbaugh & Miller 1988; Lenski & Mittler 1993; Moffat 1989; Revkin 1989).
An extension of this hypothesis suggests that the original genetic systems contained pre-programmed options susceptible to environmental induction. Perhaps organisms were originally designed with an effective mechanism for increasing genetic variability, to meet changing conditions. These mechanisms may have suffered, after that time, from mutational damage, and no longer are as effective or as reliably beneficial as they originally were. Movable elements may originally have made only regulated movements between specific sites on the chromosomes. Some such movements are still quite specific, but mutational changes in the system may have reduced their specificity.
REGULATORY GENES AND HETEROCHRONY IN EVOLUTION
A. Naturalistic evolution
Advances have been made by conventional evolutionary theory in understanding processes that can generate significant change with a minimum of genetic innovation. These processes center around changes in regulatory genes and alteration of growth processes during embryological development (see Alberch 1985; Arthur 1984; Avers 1989; Futuyma 1986; Gould 1977; McKinney & McNamara 1991; Valentine 1992; Valentine & Campbell 1975; Valentine & Erwin 1987).
The genetic material contains both structural genes that produce specific proteins and regulatory genes that control the activation of the structural genes and determine when, in what cells, and for how long, each structural gene produces its unique protein. It has been recognized that to produce significant evolutionary changes merely by a succession of mutations in structural genes would be a painfully slow and unlikely process. A different process has been suggested that relies more on changes in regulatory genes. The first step in the process would be the evolution of a great variety of structural genes, through the action of gene duplication, mutation and natural selection. When living systems contained a sufficiently diverse array of structural genes, novel body plans would result from changes in regulatory gene systems, altering the patterns of activation of the structural genes. New body plans would be primarily just new combinations of features that were already present, and consequently the establishment of these new body plans (new phyla) could proceed rapidly, in relation to geologic time (but still over thousands to millions of years).
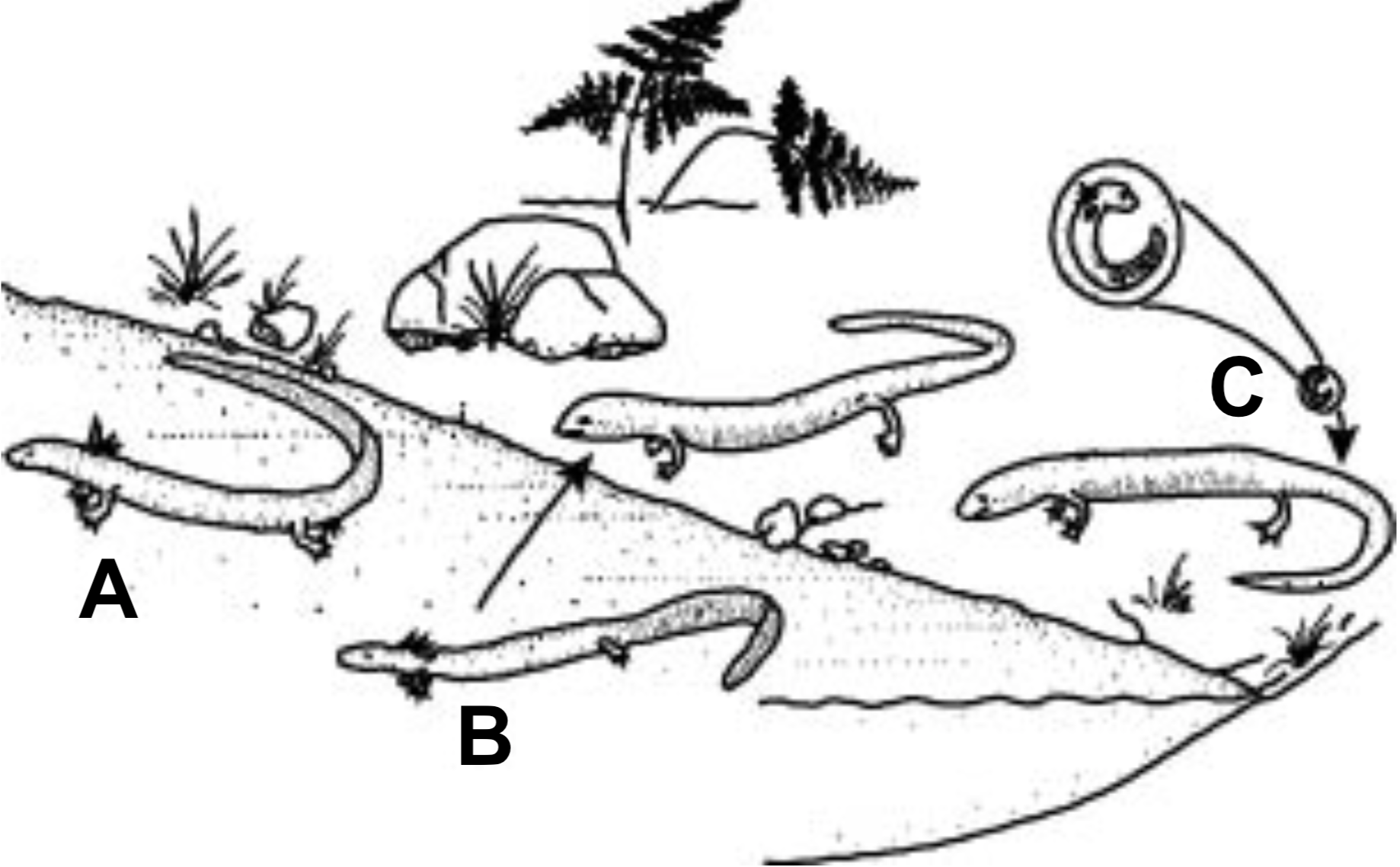
It is claimed that regulatory changes can be particularly effective if they alter the pattern of embryological development. A minimal amount of genetic change in the timing of developmental events (heterochrony) might result in significant morphological evolution. Accelerating or retarding the time of reproductive maturity (adulthood) relative to physical growth can cause quite different effects depending on the direction of the change. For example, speeding up maturation relative to physical growth can result in paedomorphosis, which is the retention of juvenile characteristics in the adult. If this results in adults with small body size, it is called progenesis; and if the adult is at least as large as its ancestral form but retains juvenile features into the adult stage, it is neoteny. Some salamanders have gills as larvae but not in the adult form. Other, neotenic species retain the gills as adults (Figure 4).
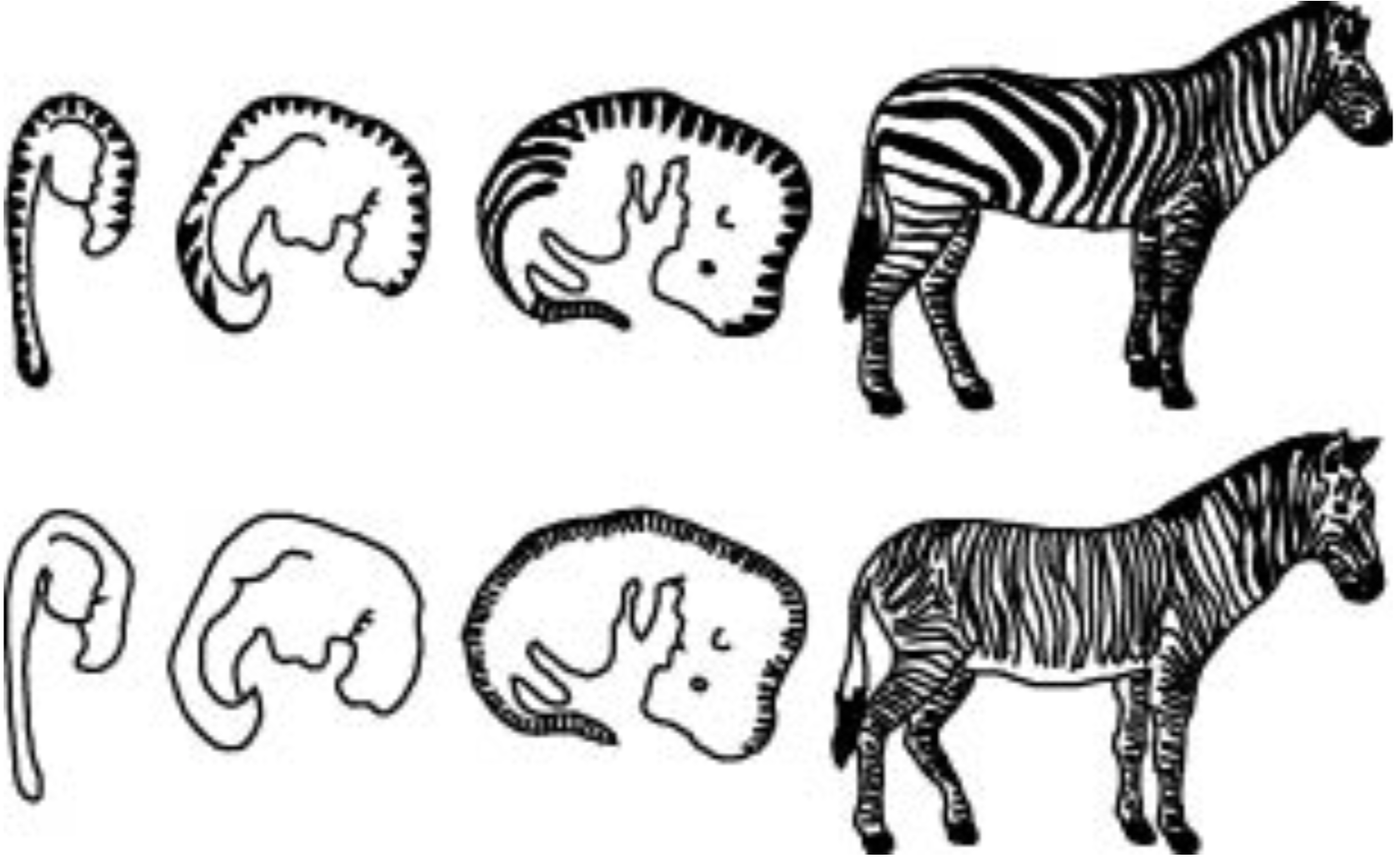
Timing of embryonic events apparently controls the stripe pattern in some zebras. The stripes on the lower back of the zebra Equus burchelli are widely and irregularly spaced, as the result of differential growth rates of the embryo after the stripe pattern is established. The stripe pattern in Equus grevyi is not established until that differential growth is completed, and consequently the stripes in the adult of this species are more equally spaced (Figure 5).
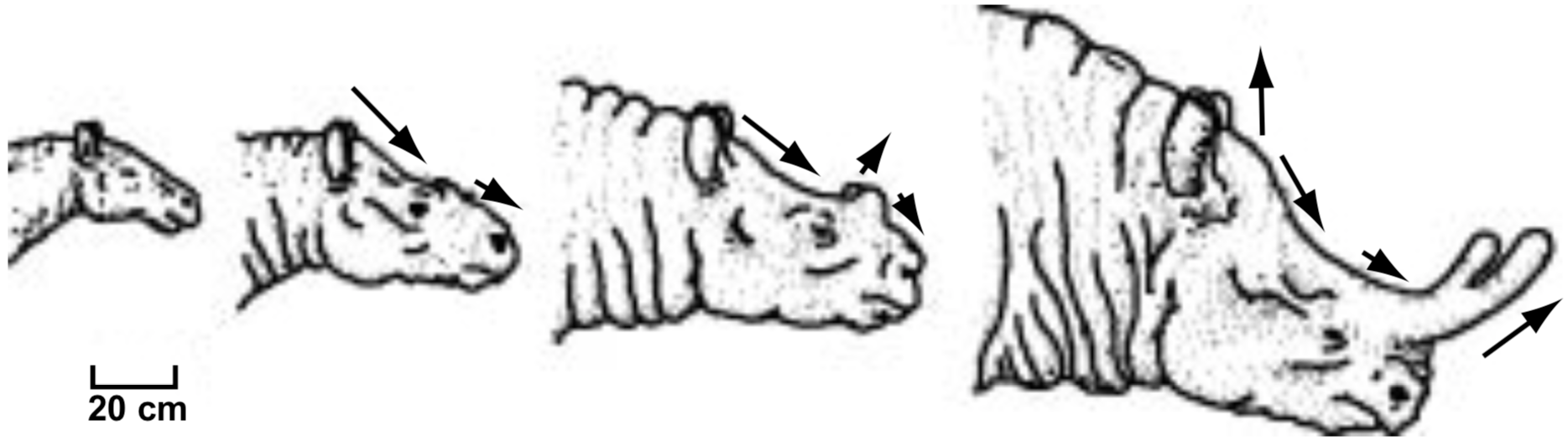
Allometry, or differential growth, has been proposed to explain the differences, for example, between several species of fossil titanotheres (Figure 6) (Futuyma 1986, p. 368). The change from one species to another is simply increased overall size, and proportionally faster growth of the horn and certain other facial features. These examples illustrate the theory of how small genetic changes over long periods of time can produce significant morphological evolution.
B. Interventionism
According to interventionist theory, the above-described processes of regulatory gene mutations and heterochrony would not produce new body plans or other major changes, but at lower taxonomic levels (for example within a family) they would perhaps help to explain how a significant amount of change could occur rapidly.
The original array of structural and regulatory genes for each body plan did not arise by mutation and natural selection, but were invented by intelligent design. The mechanisms described above are part of the process for introducing variations within each body plan, for the purpose of permitting species to adapt to changes in their environment. The gene switching model of Oster and Alberch (1982) also suggests how morphological change could occur by switching from one embryological "program" to another, with each program leading to a different morphology. Other mechanisms, which are beyond the scope of this paper, have been proposed.
SOCIOBIOLOGY
A naturalistic theory of evolution must be able to explain the origin of all animal behavior, and sociobiology claims to provide the mechanism to accomplish this. A previous article (Brand & Carter 1992) discussed sociobiology, its success in explaining many aspects of animal behavior, and its implications for human biology.
Alternative explanations for the data in this field are discussed below.
A. Naturalistic evolution
Sociobiology theory claims that the behavior of animals is biologically determined (i.e., genetically controlled) and that its evolution has been governed by the incessant evolutionary competition between genes. Complex behaviors have evolved from simple behaviors, generally from simple maintenance routines such as preening, eating, and defense. These behaviors became elaborated into more complex behaviors with new functions. Complex social behavior, including seemingly "altruistic" behavior, has evolved only as this behavior resulted in increased inclusive fitness through kin selection. In other words, an animal will perform only those behaviors that will maximize the passing on of its genes, either through its own survival and reproduction or by assisting the survival and reproduction of its relatives who share many of the same genes that it has. Thus there is no truly altruistic behavior; behavior that appears altruistic has evolved only because it serves the interests of the "selfish genes" and has increased the potential of these genes to be passed on to more offspring.
B. Interventionism
According to interventionist theory the original animals had the greatest level of complexity in their behavior, and the interspecific and intraspecific interactions between organisms were the most finely tuned and harmonious at the beginning of life on Earth. Potential conflicts between animals over the division of territory and other resources were originally settled by non-damaging conventional displays like those still common in a number of animals. Examples include the male rattlesnake wrestling matches and the lizard tail lashing or head butting "battles." True altruistic behavior may have been much more common. Perhaps it was originally common for subadult animals to assist their parents in raising the next brood or litter. Population control mechanisms were also much more finely tuned than at present. Behavioral mechanisms for maintaining a stable ecological balance were built into the animals' genetic makeup; part of an ecological system that originated through intelligent design rather than chance.
The instinctive behavioral mechanisms which prevented damaging conflict were not originally subject to random mutational changes. Because of adequate protection from mutational damage, individuals with these behavioral mechanisms would not be subject to unfavorable competition from individuals who would benefit from behavioral "cheating." With the introduction of random mutations these behavioral mechanisms began to break down.
Natural selection, and especially kin selection, has acted to slow this breakdown. The altruistic behaviors which have survived the negative effects of mutation are primarily those that have been preserved by kin selection and that increase the inclusive fitness of the organism. When mutations began to cause the loss of some of the original created behavior patterns, natural selection would inevitably come into play and determine whether the original type or the mutated type would become most common. If mutations in a female bird removed the original pattern of helping her parents raise their young, and she built her own nest, she would likely produce more young in her lifetime than other young who began reproducing later (this is the same result that would be expected by naturalistic theory). As a consequence the "non-helper genome" would become more common and eventually replace the "helpers." On the other hand, in some situations the genes for "altruistic" behavior are favored by kin selection, and consequently will continue to be common in the population. The Florida Scrub Jay lives in a situation in which the young are not likely to successfully reproduce the first year, and consequently their inclusive fitness will be increased if they help their parents raise young which share many genes that they also have. In this way more copies of their genes will exist in the population than if they didn't help their parents that first year, and thus kin selection favors retention of the "altruistic" behavior in this environment.
Mutation and natural selection have no ability to look at the "big picture" and see what is best for the overall ecological balance. Natural selection is strictly shortsighted it favors any change that increases successful reproduction. The ultimate result of the rule of natural selection in nature is the competitive, vicious side of nature.
CONCLUSION
This interventionist theory has a number of implications for the genetic system, along with suggestions for future research. An obvious implication is that with adequate genetic variability and changing environments, morphological change and speciation can occur rapidly, even orders of magnitude faster than is commonly believed. Animal populations that are well adapted to their environment would not be expected to change, but rapid evolution within limits is seen as the normal expectation under some environmental conditions, especially when rapid environmental changes are occurring.
We propose that evolutionary change has occurred only within definite limits, but the limits are not at the species level. Because of the subjectivity involved in defining higher categories in different animal groups, it will not be possible to define the limits of the original groups of animals and plants in terms of a specific taxonomic level such as family or genus, but preliminary analysis suggests to us that almost all modern species, probably most modern genera and perhaps some families, have resulted from modifications of the originally created species.
These changes involved mutations and natural selection, loss of some information, and adaptation to changing environments. Changes in regulatory genes have probably been an important factor making rapid change possible, since small genetic changes produce relatively large phenotypic effects. Could even the series of titanotheres and horses have resulted from these processes?
Naturalistic theory proposes that the existing structural genes accumulated through the action of mutation, recombination, and natural selection. The process is believed to have been facilitated by duplication of genes, producing excess genetic material that could then be modified by mutations, eventually becoming new genes coding for new proteins. Much of the genetic material in organisms consists of "silent DNA" with no known function. Part of this DNA contains pseudogenes, which appear to be copies of known genes, but with mistakes in them. Pseudogenes and other silent DNA are usually interpreted as duplicated genes that can evolve into new genes.
It is being recognized that more of the "junk DNA" is functional then had previously been thought (Nowak 1994; Reynaud et al. 1989). We think that this trend will continue, and it will be found that much more DNA is involved in regulation than is currently recognized. When we consider all of the control mechanisms needed to regulate when and where each protein will be made and in what quantity; the development of each different organ and its growth and integration with other organs; the functioning of the tremendously complex biochemical systems in each cell, as well as controlling how long your nose will be, it becomes evident that a vast complex of regulatory genes is needed. There are certainly many more regulatory genes than structural genes, and we predict that in most organisms, the amount of DNA needed for structural and regulatory genes is much greater than presently recognized.
However, we cannot rule out the likelihood that in some cases mutations may have produced extra copies of genes. For example, it is puzzling why the amount of DNA per organism varies by two orders of magnitude in fish and in insects, and by three orders of magnitude in algae and in angiosperms (John & Miklos 1988, p. 150).
Is it actually possible for complexes of structural and regulatory genes to originate through mutation, recombination, and natural selection? This requires that the duplicated DNA gradually accumulate beneficial changes that can be selected for, and that this process can produce a new gene with a new function. Is it possible for this to occur with no intelligent input, producing not only a new structural gene but also the complex of regulatory genes that recognize and control it? We predict that the answer is no.
It has been proposed that the evolution of resistance to insecticides, and new enzymes appearing in laboratory cultures of bacteria, etc., are examples of this process. As our understanding of the details of the genetic material improves, along with more effective techniques for analyzing it, it should become possible to test the theory that mutation and natural selection can produce new genes. Are those actually new enzymes that appear in bacteria cultures, or just the activation of genetic potential that was already there but not in use (or at a low level) before the environment was changed (Opadia-Kadima 1987)?
Research should focus on determining the exact genetic information in organisms used in the research described above so that it will be known whether new genes actually appear by the hypothesized process. Perhaps it would also be possible to induce sufficiently accelerated mutation rates to attempt to duplicate the gene evolution process in the laboratory. Efforts are also being made to develop computer simulations of genetic systems (Maynard Smith 1992). As our understanding of genetic mechanisms improves, perhaps the sophistication of such models could become adequate to realistically test theories of gene evolution.
It seems most likely to us that any process of genetic change which depends on random mutations as the ultimate source of new information will tend to produce disorder, and will never construct any new gene complexes. Until that prediction can be falsified, the theory of naturalistic megaevolution of higher categories from a common ancestor stands on a weak foundation.
The evidence suggests to us that quite a bit of speciation and morphological change has occurred, and the reinterpretation of evolutionary genetics presented here is proposed as a step toward understanding the process of change that brought life from the original created state to its present adaptation to modern conditions. We propose that these genetic mechanisms are adequate only to diversify and adapt life from the original created taxa, and cannot produce an increase in the complexity of life. The evidence for a genetic mechanism adequate to produce increased complexity and new body plans is far from compelling.
REFERENCES CITED
- Alberch, P. 1985. Problems with the interpretation of developmental sequences. Systematic Zoology 34:46-58.
- Amabile-Cuevas, C. F., and M. E. Chicurel. 1993. Horizontal gene transfer. American Scientist 81:332-341.
- Arthur, W. 1984. Mechanisms of morphological evolution. John Wiley, New York.
- Ashton, E. H., R. M. Flinn, and R. K. Griffiths. 1979. The results of geographic isolation on the teeth and skull of the green monkey (Cercopithecus aethiops sabaeus) in St. Kitts a multivariate retrospect. Journal of Zoology, London 188:533-555.
- Avers, C. J. 1989. Process and pattern in evolution. Oxford University Press, New York.
- Baker, A. J. 1987. Rapid genetic differentiation and founder effect in colonizing populations of common mynas Acridotheres tristis). Evolution 41:525-538.
- Bakker, R. T. 1985. Evolution by revolution. Science 85:72-80.
- Barash, D. 1974. The evolution of marmot societies: a general theory. Science 185:415-420.
- Brand, L. R. and R. L. Carter. 1992. Sociobiology: the evolution theory's answer to altruistic behavior. Origins 19:54-71.
- Cain, A. J. 1989. The perfection of animals. Biological Journal of the Linnaean Society 36:3-29.
- Cairns, J., J. Overbaugh, and S. Miller. 1988. The origin of mutants. Nature 335:142-145.
- Carson, H. L. 1975. The genetics of speciation at the diploid level. American Naturalist 109:83-92.
- Carson, H. L. and R. G. Wisotzkey. 1989. Increase in genetic variance following a population bottleneck. American Naturalist 134:668-673.
- Dessauer, H. C., G. F. Gee, and J. S. Rogers. 1992. Allozyme evidence for crane systematics and polymorphisms within populations of sandhill, sarus, Siberian and whooping cranes. Molecular Phylogenetics and Evolution 1:279-288.
- Diamond, J. M. 1981. Flightlessness and fear of flying in island species. Nature 293:507-508.
- Dilger, W. C. 1960. The comparative ethology of the African parrot genus Agapornis. Zeitschrift fur Tierpsychologie 17(6):649-685.
- Dilger, W. C. 1962. The behavior of lovebirds. Scientific American 206:88-98.
- Dodson, E. O. and P. Dodson. 1985. Evolution: process and product. PWS Publishers, Boston, Massachusetts.
- Eiseley, L. C. 1979. Darwin and the mysterious Mr. X. Harcourt Brace Jovanovich, New York.
- Endler, J. A. 1986. Natural selection in the wild. Princeton University Press, Princeton, New Jersey.
- Fontdevila, A. 1992. Genetic instability and rapid speciation: are they coupled? Genetica 86:247-258.
- Ford, E. B. 1964. Ecological genetics. John Wiley, New York.
- Futuyma, D. J. 1986. Evolutionary biology. 2nd ed. Sinauer Associates, Inc., Sunderland, Massachusetts.
- Gould, S. J. 1977. Ontogeny and phylogeny. Belknap Press of Harvard University Press, Cambridge, Massachusetts.
- Hinegardner, R. 1976. Evolution of genome size. In F. J. Ayala (ed.), Molecular Evolution, pp. 179-199. Sinauer Associates, Inc., Sunderland, Massachusetts.
- Ho, M. W. and P. T. Saunders. 1979. Beyond neo-Darwinism an epigenetic approach to evolution. Journal of Theoretical Biology 78:573-591.
- John, B. and G. L. G. Miklos. 1988. The eukaryote genome in development and evolution. Allen and Unwin, Boston, Massachusetts.
- Johnson, M. W. 1953. The copepod Cyclops dimorphus Kiefer from the Salton Sea. American Midland Naturalist 49:188-192.
- Johnston, R. F. and R. K. Selander. 1964. House sparrows: rapid evolution of races in North America. Science 144:548-550.
- Langridge, J. 1987. Old and new theories of evolution. In K. S. W. Campbell and M. F. Day (eds.), Rates of Evolution, pp. 248-262. Allen and Unwin, London.
- Lenski, R. E. and J. E. Mittler. 1993. The directed mutation controversy and neo-Darwinism. Science 259:188-194.
- Lester, L. P. and R. G. Bohlin. 1989. The natural limits to biological change. 2nd edition. Probe Books, Word Publishing, Dallas, Texas.
- Løvtrup, S. 1987. Darwinism: the refutation of a myth. Croom Helm, New York.
- McKinney, M. L. and K. J. McNamara. 1991. Heterochrony: the evolution of ontogeny. Plenum Press, New York.
- Maynard Smith, J. 1989. Evolutionary genetics. Oxford University Press, New York.
- Maynard Smith, J. 1992. Byte-sized evolution. Nature 355:772-773.
- Mayr, E. 1970. Populations, species, and evolution. Belknap Press, Cambridge, Massachusetts.
- Mettler, L. E., T. G. Gregg, and H. E. Schaffer. 1988. Population genetics and evolution. Prentice Hall, Englewood Cliffs, New Jersey.
- Moffat, A. S. 1989. A challenge to evolutionary biology. American Scientist 77:224-226.
- Nowak, R. 1994. Mining treasures from "junk DNA." Science 263:608-610.
- Opadia-Kadima, G. Z. 1987. How the slot machine led biologists astray. Journal of Theoretical Biology 124:127-135.
- Oster, G. and P. Alberch. 1982. Evolution and bifurcation of developmental programs. Evolution 36:444-459.
- Parsons, P. A. 1987. Evolutionary rates under evolutionary stress. Evolutionary Biology 21:311-347.
- Parsons, P. A. 1988. Evolutionary rates: effects of stress upon recombination. Biological Journal of the Linnaean Society 35:49-68.
- Reanna, D. C. 1985. The origin, nature and significance of genetic variation in prokaryotes and eukaryotes. In K. S. W. Campbell and M. F. Day (eds.), Rates of Evolution, pp. 235-247. Allen and Unwin, Boston, Massachusetts.
- Revkin, A. C. 1989. March of the fire ants. Discover 10(3):71-76.
- Reynaud, C., A. Dahan, V. Anquez, and J. Weill. 1989. Somatic hyperconversion diversifies the single VH gene of the chicken with a high incidence in the D region. Cell 59:171-183.
- Ridley, M. 1993. Evolution. Blackwell Scientific Publications, Boston, Massachusetts.
- Simpson, G. G. 1953. The major features of evolution. Columbia University Press, New York.
- Terzian, C. and C. Biemont. 1988. The founder effect theory: quantitative variation and mdg-1 mobile element polymorphism in experimental populations of Drosophila melanogaster. Genetica 76:53-63.
- Valentine, J. W. 1992. The macroevolution of phyla. In J. H. Lipps and P. W. Signor (eds.), Origin and Early Evolution of the Metazoa, pp. 525-553. Vol. 10 in Topics in Geobiology (F. G. Stehli and D. S. Jones, series eds.). Plenum, New York.
- Valentine, J. W. and C. A. Campbell. 1975. Genetic regulation and the fossil record. American Scientist 63:673-680.
- Valentine, J. W. and D. H. Erwin. 1987. Interpreting great developmental experiments: the fossil record. In R. A. Raff and E. C. Raff (eds.), Development as an Evolutionary Process, pp. 71-107. Liss, New York.
- Zimmerman, E. C. 1960. Possible evidence of rapid evolution in Hawaiian moths. Evolution 14:137-138.