©Copyright 2018 GEOSCIENCE RESEARCH INSTITUTE
11060 Campus Street • Loma Linda, California 92350 • 909-558-4548

LIFE IN THE DEEP ROCKS AND THE DEEP FOSSIL RECORD
by
Ariel A. Roth
Geoscience Research Institute
WHAT THIS ARTICLE IS ABOUT
It has been known for many years that microorganisms can exist in rocks several kilometers below the surface of the earth. Recently a number of reports indicate that these organisms are much more common than previously surmised and that vast regions of the underworld may be inhabited.
This new information has interesting implications for both evolutionism and creationism. From the evolution viewpoint, simple organisms, whose poorly preserved fossils are found in the older rocks, represent early stages of evolution. Could these represent not-so-old organisms that had been living in the rocks? For creation the new findings can suggest that the fossils found in these lower rocks represent life in the rocks that existed there since a recent creation. The similarity of some of the fossil forms to modern ones lends credence to this concept.
LIFE IN THE ROCKS
We are all familiar with the animals and plants on land, as well as plankton, fishes and whales of the world oceans; however, a new biological realm is coming into focus: that of life in the rocks. The rocks of the crust of the earth, especially the deeper ones, are relatively inaccessible. "Out of sight out of mind" certainly applies here; and it is not surprising that although we have known of some life in deep rocks for decades, only recently have scientists given serious attention to this hidden biological realm.
It has long been known that organisms such as bacteria, worms and insect larvae abound in the top 1 m (3 ft) of Earth's soils. Below this level, the number of organisms decreases dramatically, but persists to great depths in surprising numbers. Microorganisms of various kinds are the only kind of life that flourishes at these depths. Examples abound. [1] Sulfur-reducing bacteria are abundant in aquifers 800-1000 m deep in the Bachu district (former USSR). In that region bacteria are so abundant they impart a pink color to water coming from oil-well drilling. One well produced some 5000 kg (11,000 lbs, or 1400 gal) of pink water daily for 6 months. [2]
In England, iron-and sulfur-oxidizing bacteria produce a red slime found in abundance in a tin mine located in granite rock at a depth of 600 in (2000 ft). [3] A coal seam in Germany harbors about 1000 bacteria per gram of coal lying at a depth of 400 m (1300 ft). About the same concentration of bacteria was found in groundwater over 1000 m (3300 ft) below the surface, in the Madison limestone of the northwest U.S.A. [1]
Bacteria can readily grow when introduced into deep environments. Some that oxidize methane have been injected into coal layers to significantly reduce the concentration of that explosive gas in coal mines. Bacteria are also being used to enhance oil production by releasing oil from sedimentary reservoirs. [2]
Extensive studies have been conducted in South Carolina in three boreholes, with depths as great as 500 m (1600 ft). Typically 100,000 to 10,000,000 bacteria were found per gram of sediment, and over 4500 different strains were isolated. In less permeable sedimentary layers (clay) lying between aquifers the numbers of bacteria were much fewer typically less than 1000 per gram. [4] Protozoa (one-celled animals) and fungi were also found, but in significantly lower concentrations than bacteria. [5] Protozoa and bacteria have also been found in a number of other deep subsurface sediments. [6] Surprisingly, at the South Carolina site, unicellular and filamentous live green algae that usually require light for growth were found at a number of levels in two of the boreholes down to 210 m (700 ft). [5] Their presence at these great depths was explained as possibly indicating some sort of connection to the surface, or a very long viability for these algae. Another study demonstrated the presence of viruses of the bacteriophage type at a depth of 405 m (1330 ft). [7]
Microorganisms are probably found in all sedimentary rocks, [8] and are most abundant in aquifers. They have also been discovered in granite. Thomas Gold [9] provides convincing evidence of their activity at a depth of 6000 m (20,000 ft) in an exploration oil well drilled in Sweden's Siljan impact crater (44 km, or 27 mi, diameter). Furthermore, he reports on the isolation of several strains of living bacteria found in depths greater than 4000 m (13,000 ft) at the same locality. He even suggests that the volume of living organisms in the rocks may be comparable to that of all organisms living on the surface of the earth. [10] Considering the thickness of the rock layers, one can envision a lot of life below our feet.
The abundance of life in the rocks has rekindled interest in life on Mars. In some quarters, it is hoped that life can be found in the deep rocks of that planet. Future robotic and human-piloted missions to Mars should incorporate strategies to test this. [11]
Part of the success of microorganisms in the rocks is due to their very small size, permitting them to exist in very small pore spaces. Bacteria are commonly around 1 mm (1/1000 mm, or 1/25,000 in) in diameter or length. Protozoa, algae, fungi and cyanobacteria (bacteria that have photosynthetic capability) are generally 10-100 times larger, but still are an easy fit between particles of coarser sediments such as sands. Moisture is essential for their survival, but water is common in many areas down to 1 km (0.6 mi), and often many times that depth. The slow lateral and vertical transport of water in aquifers favors the passive spread of microorganisms.
Recently it has been discovered that these microorganisms can attack rocks, probably using the organic acids they secrete. This kind of activity is enhanced in the presence of an organic source such as oil. [12] They can also precipitate certain minerals and may thus be insidious sculptors of the subterranean environment, opening and closing groundwater flow-paths. [13] This ability to attack rock is a matter of major concern if radioactive waste is stored in rocks. If this waste and surrounding rocks are attacked by microorganisms, there might be consequent radioactive contamination of groundwater. [3] In shallower environments these microorganisms cause considerable commercial damage, aiding in the corrosion of metals. Rusted and failed pipelines are a problem of massive proportions. In England alone damage is estimated at half a million pounds per year. [14]
The various organisms found at depths possess a multitude of biochemical systems that permit them to survive under unusual conditions. Many require oxygen while others cannot survive in its presence. Others can go either way. Often there is a moderate amount of oxygen in the waters found at these depths, while pockets with no oxygen are not uncommon. Energy is obtained from both organic and inorganic compounds, and a number of ingenious metabolic mechanisms are being discovered.
Often these organisms can survive at unusually high temperatures which are common at these depths. Many thrive at temperatures well above the boiling point for water at sea level (100ºC, 212ºF). At great depths, high ambient pressures keep the water from boiling and provide a fluid, but nevertheless very hot, environment. It is commonly surmised that these organisms could survive at temperatures up to 150ºC (300ºF). The higher temperatures found in rocks at depths beyond a few kilometers would exclude life at such depths. However, the successful culture of bacteria obtained from "black smoker" sulphide chimneys deep in the Pacific Ocean at 250ºC (480ºF) under 265 atmospheres of pressure has been reported. [15] Interestingly, some of the hot springs from the deep floor of the ocean extrude living bacteria in concentrations as high as a billion per milliliter of water.
From the above it is obvious that there is a previously unknown world of life dwelling in the rocks that should be further investigated. Unfortunately these secretive organisms are relatively inaccessible. Their presence poses some interesting questions regarding the fossil record of microorganisms as found in the deeper rocks.
THE GEOLOGIC COLUMN
Recently evolutionists have been placing special emphasis on fossil finds of simpler life among what is considered to be the earliest rocks of the earth. A review of some of the more important findings as they relate to the geologic column will help in elucidating interpretations.
The major divisions of the geologic column are outlined in Table 1. One can think of these layers as being superimposed, with the oldest being at the bottom. Actually, each of these divisions can be found today on Earth's surface, with the lower ones being exposed by uplift and erosion. The lowest layers have been studied intensively by paleontologists in their search for clues about the earliest forms of evolving life on earth.
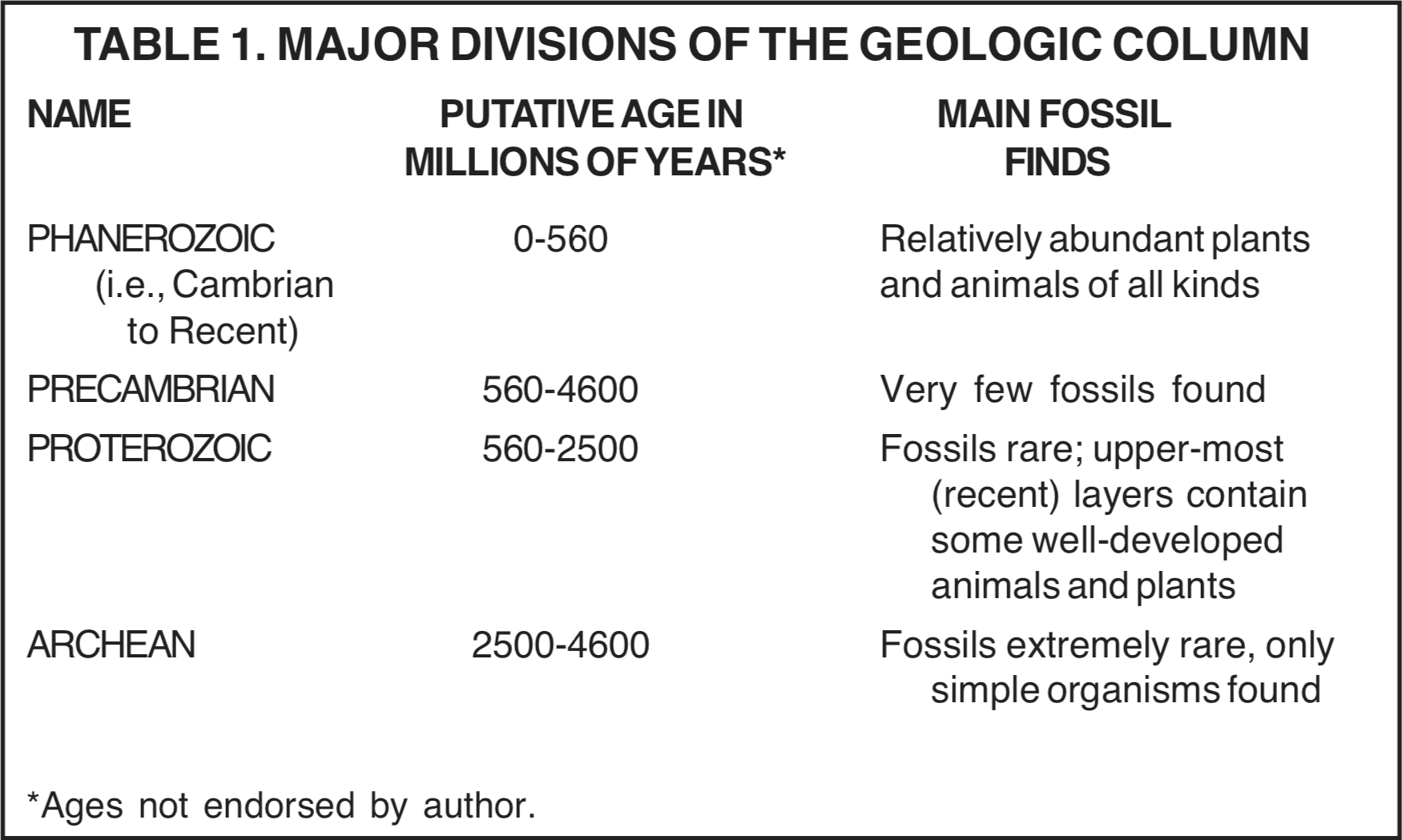
A number of fossil unicellular organisms have been described for the Archean (see Table 1). Study has concentrated on the Swaziland Supergroup of South Africa and the Warrawoona Group near North Pole (so-called because, like the real North Pole, it is a very desolate area) in Australia. From each of these regions, both filamentous types of fossils [16] and stromatolites have been described. Stromatolites are finely layered sedimentary structures, generally of centimeter to meter size, usually in a domed or wavy shape. They are formed by living organisms, mainly bacteria, that live on the surface of the stromatolite. The bacteria, which usually require light, function in the capture and/or precipitation of sediments that gradually build up the stromatolite.
In the Proterozoic (see Table 1), stromatolites are relatively abundant, especially in the lower part. Special mention should be made of the Gunflint Chert of the Great Lakes region of the U.S. This chert, also from the lower part of the Proterozoic, has well-preserved filamentous fossils that look very much like the modern Oscillatoria cyanobacterium (blue-green algae). [17]
Peculiar spherical organisms called acritarchs, which are commonly 50 mm (0.002 in) in diameter and thought to be some form of algal cysts, [18] are found in the upper half of the Proterozoic. They show great diversity and size increase near the top. These are the first generally accepted evidence for organisms with cells containing a nucleus; however, the evidence has been disputed. Organisms with cells that have a nucleus are called the Eukaryotes. These include most kinds of living organisms from protozoa to redwood trees. By contrast, bacteria which have no nucleus are called prokaryotes. Several other fossil types have been described for the Proterozoic, including peculiar small vase-shaped objects (70 mm, 0.003 in) of unknown affinity.
In the very top of the Proterozoic are found mostly unfamiliar Ediacaran multicellular types of animals. No multicellular animals have been found below this level. Directly above this level is the so-called "Cambrian Explosion" at the base of the Phanerozoic (see Table 1), where the majority of the basic kinds of animals first appear. The scarcity of fossils in the Precambrian is well illustrated by the fact that during the previous century no fossils were found in that portion of the rock layers. Recently the situation has changed.
THE PROBLEM OF FOSSIL IDENTIFICATION
Determining whether a peculiar form in a rock is a bona fide fossil can be difficult. Curls caused by the desiccation of sediments have been interpreted as arthropod parts; drag marks caused by storms can resemble worm tracks; and pyrite rosettes have been interpreted as medusae (jellyfish). [19] The terms pseudofossils and dubiofossils are used to describe false or dubious fossils.
Intensive search by paleontologists for early life has produced many suggested candidates, but authentication is a problem. Many non-biogenic structures can simulate the general shape and characteristics of these assumed early simple cells. Additionally, by simple inorganic chemical precipitation, several workers have succeeded in producing spherical and tube-like structures that highly resemble what is being described as evidence of life in these early layers. [20] It is to the credit of paleontologists that recently a considerable amount of caution is being expressed regarding the authenticity of most of the findings in the early Precambrian rocks. Schopf and Packer, in referring to microfossils reported from at least 28 Archean geologic units, state: "However virtually all have recently been reinterpreted as dubiofossils or as nonfossils: pseudofossils, artifacts, or contaminants." [21] Cowen states: "Only a few reports of fossil Archean cells seem to be genuine out of fifty or more claims." [22] Buick has pointed out a host of problems with the identification of most fossil finds from North Pole, Australia. [23]
Stromatolites have not fared much better. The question is: are they formed biologically or are they just the passive accumulation of fine layers of sediments, possibly subjected to some deformation? Ginsburg points out that "Almost everything about stromatolites has been, and remains to varying degrees, controversial." [24] Hoffman notes: "Something that haunts geologists working on ancient stromatolites is the thought that they may not be biogenic at all." [25] He illustrates this with the notorious example of the "algal pisolites" (rock composed of pen-size spheres) of the Permian in Western Texas which were thought to have been formed biologically in a similar way to stromatolites, but turned out to be of inorganic origin. [26] The well-known paleontologist Charles Walcott, who for twenty years was Director of the Smithsonian, described 5 new genera and 8 new species of strornatolites which he believed to be of biological origin. All have since been reinterpreted as inorganic by some workers. [27] Interestingly, no cells have been found associated with any Archean stromatolites.
The question of the temporal significance of stromatolites is further complicated by the recent discovery of living stromatolites forming in cavities in rocks such as in coral reefs. These are called endostromatolites. Sediment accumulation would be facilitated by bacteria that do not require light as an energy source. Furthermore, Monty suggests that endostromatolites can form in rock cavities at depths of at least 3000 m (10,000 ft) below Earth's surface. [28] This raises the question as to whether some stromatolites in the Precambrian may actually be endostromatolites of much more recent origin.
Attempts have been made to validate the authenticity of Precambrian fossils by testing for the isotope fractionation of carbon and sulfur that would be expected from biological activity. Some positive results have been obtained, but Buick [23] rejects these outright, since controls are too variable. Knoll [29] comments about little fractionation in sulfur, and Nagy et al. [30] give good evidence of contamination of sediments assumed to be very old by molecules originating from recent organisms.
Despite all the problems in identifying Precambrian fossils, it appears that there are still a few good examples. They include the Gunflint Chert cyanobacteria, the acritarchs, the Bitter Springs cyanobacteria and the Ediacaran animal fauna, all of which are Proterozoic.
SIGNIFICANCE TO THE EVOLUTION VIEWPOINT
Evolutionists have sometimes suggested that the first organisms to evolve were closely related to the sulfur bacteria mentioned above. [10] These are part of a group called the Archeabacteria. Later the true bacteria or Eubacteria are assumed to have evolved from the Archeabacteria, and they developed photosynthetic and stromatolite-building capabilities. The more-advanced forms of life with nuclei in their cells the Eukarya are considered to have evolved later. This scenario has been challenged by studies of molecular phylogenies which show evolutionary relationships on the basis of sequential similarities in large organic molecules. Ribosomal RNA is a favorite. It turns out that the two basic bacterial types the Archeabacteria and the Eubacteria, which are both simple cells without a nucleus and look very similar to each other, are as far apart from each other in terms of ribosomal RNA differences as all the rest of the living organisms combined (i.e., from protozoa to redwood trees), which are all Eukaryotes (cells with a nucleus). This surprising result has caused evolutionists to propose that all three groups Archeabacteria, Eubacteria, and Eukaryotes evolved very early so as to give equal time for differentiation of the ribosomal RNA molecules according to the molecular-clock hypothesis. [29] This newer evolutionary concept challenges both the older concept of ancestral Archeabacteria and the findings of the fossil record. Good examples of Eukarya do not appear until the middle of the Proterozoic, and the evidence there is not very certain. [31] On the other hand, in accord with the theory, filamentous Eubacteria are assumed to have existed as far back as the middle of the Archeozoic, [16] and stromatolites are also described there. Hence the molecular clock and the fossil record do not appear to be in synchrony. One could explain this by proposing that the early Eukaryotes were different from modern types and have not been recognized, but more evidence is needed.
One wonders if the newer information regarding the abundance of life in rocks might not modify evolutionary interpretations. Some questions have been raised regarding the primary nature (i.e., are the fossils part of the original deposit?) of a number of Archeozoic fossil finds, [23] but thus far, to this writer's knowledge, the significance of organisms living in rocks as more recent contaminants has not received any attention from proponents of the evolution viewpoint. This evidence has the potential for challenging views that the Precambrian fossils represent ancient simple forms of life in the early stages of evolutionary development.
SIGNIFICANCE TO THE CREATION VIEWPOINT
Creationists have paid little attention to the Precambrian. Traditionally, because of the paucity of fossils, Precambrian sediments have been considered to be deposits made before the Genesis flood. Recent information regarding Precambrian fossils has prompted some reinterpretation. Snelling [32] suggests the Precambrian sediments represent flood deposits, while Wise [33] proposed that the Precambrian fossils represent organisms created on Day 2 of creation week and buried on Day 3. Each of these views deserves further consideration.
I would like to suggest that the Precambrian fossils (except for the Ediacaran metazoa which are very close to the Cambrian) might originate from two sources. 1) Normal life in the rocks as is being found now, and existing at any time since creation. These could be pre-flood, flood, or post-flood in origin. 2) Local infiltration into Precambrian rocks resulting from the upheaval of the Genesis flood. Such an event would be expected to facilitate the inflow of water and organisms along cracks and fault lines into Precambrian rocks. For each of these sources, Precambrian fossils originate from a recent creation and do not reflect evolutionary development. The Ediacaran animal fossils of the uppermost Precambrian would be considered a flood deposit.
The concept of life in the deep rocks before the flood adds a new dimension for the ecological zonation model of the fossil sequence. [34] That model proposes that the sequence of fossils now found reflects the pre-flood ecology. Under this concept the living organisms in the deep pre-flood rocks would be the source of the fossils we now find in the Precambrian.
One piece of evidence, supportive of a recent origin for Precambrian fossils, deserves mention: the very close similarity of some of the Precambrian fossils to present living forms. Their similarity seems unusual if they have had two billion (2 × 109) years to evolve. Stewart comments on the Bitter Springs cherts of central Australia:
Many more examples could be given to emphasize the similarity of the fossils and extant floras which is so striking that one has to wonder about the slow rate of evolution among the Cyanophyta for the last 900 m.y. [million years]. [35]
Schopf reports on several fossil species in this formation that appear identical to present living species. [36] Some forms in the Gunflint Chert, which is assumed to be nearly two billion years old, are also very similar. Speaking more generally, Knoll states:
Many Late Proterozoic prokaryotes differ little in morphology, development, or behaviour from living cyanobacterial populations. [37]
Evolutionists try to explain this lack of change on the basis of an episodic rate of evolution, but these similarities may well represent organisms created recently and found in rocks as part of the living underworld.
CONCLUSIONS
The recent discoveries concerning life in the deep rocks, including algal filaments at 200 m (650 ft) depth, open a whole new field for reinterpretation of the Precambrian record of simple organisms. The problematic strornatolites may represent only deformed sediments or even endostromatolites formed in deep rocks. It is proposed that the small Precambrian fossils (except for those near the upper boundary) could have come from either recently created organisms living in these rocks, or infiltration of these organisms into these lower rocks during the Genesis flood. The presence of abundant microbial life deep in the rocks challenges evolutionists with the necessity of testing the hypothesis that Precambrian microorganisms are recent contaminants, rather than 560 to 3500 million-year-old fossils.
ENDNOTES
[1](a) Ghiorse, W. C. and J. T. Wilson. 1988. Microbial ecology of the terrestrial subsurface. Advances in Applied Microbiology 33:107-172; (b) Fliermans, C. B. and T. C. Hazen (eds.). 1990. Proceedings of the First International Symposium on Microbiology of the Deep Subsurface. WSRC Information Service Section Publication Group.
[2]Ivanov, M. V. 1990. Subsurface microbiological research in the USSR. In Fliermans and Hazen, pp. 1.7-1.15.
[3]West, J. M. 1990. Subsurface microbiological research in the United Kingdom. In Fliermans and Hazen, pp. 1. 16-1.23.
[4]Balkwill, D. L. 1990. Density and distribution of aerobic, chemoheterotrophic bacteria in deep southeast coastal plain sediments at the Savannah River Site. In Fliermans and Hazen, pp. 3.3-3.13.
[5](a) Sinclair, J. L. 1990. Eukaryotic microorganisms in subsurface environments. In Fliermans and Hazen, pp. 3.39-3.51; (b) Sinclair, J. L. and W. C. Ghiorse. 1989. Distribution of aerobic bacteria, protozoa, algae, and fungi in deep subsurface sediments. Geomicrobiology Journal 7:15-31.
[6]Sinclair, J. L. and W. C. Ghiorse. 1987. Distribution of protozoa in subsurface sediments of a pristine groundwater study site in Oklahoma. Applied and Environmental Microbiology 53(5):1157-1163.
[7]Bradford, S. M. and C. P. Gerba. 1990. Isolation of bacteriophage from deep subsurface sediments. In Fliermans and Hazen, p. 4.65.
[8]Ourisson, G. 1984. The microbial origin of fossil fuels. Scientific American 251(2):44-51.
[9]Gold, T. 1991. Sweden's Siljan ring well evaluated. Oil & Gas Journal 89(2):76-78.
[10]Gold, T. 1992. The deep, hot biosphere. Proceedings of the National Academy of Sciences 89:6045-6049.
[11]Boston, P. J. and C. P. McKay. 1990. The deep subsurface of Mars: possible habitat for extant or recently extinct microbial life. In Fliermans and Hazen, p. 4.97.
[12]Hiebert, F. K. and P. C. Bennett. 1992. Microbial control of silicate weathering in organic-rich ground water. Science 258:278-281.
[13]Appenzeller, T. 1992. Deep-living microbes mount a relentless attack on rock. Science 258:222.
[14]Hamilton, W. A. 1985. Sulphate-reducing bacteria and anaerobic corrosion. Annual Review of Microbiology 39:195-217.
[15]Baross, J. A. and J. W. Deming. 1983. Growth of 'black smoker' bacteria at temperatures of at least 250ºC. Nature 303:423-426.
[16](a) Schopf, J. W. and B. M. Packer. 1987. Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science 237:70-73; (b) Walsh, M. M. and D. R. Lowe. 1985. Filamentous microfossils from the 3,500-Myr-old Onverwacht Group, Barberton Mountain Land, South Africa. Nature 314:530-532.
[17]Stewart, W. N. 1983. Paleobotany and the evolution of plants. Cambridge University Press, Cambridge, London and New York, p. 30.
[18]Mendelson, C. V. 1993. Acritarchs and prasinophytes. In J. H. Lipps (ed.), Fossil Prokaryotes and Protists, pp. 77-104. Blackwell Scientific Publications, Boston, Oxford and London.
[19]Cloud, P. 1973. Pseudofossils: a plea for caution. Geology 1(3):123-127.
[20](a) Pickett, J. and V. Scheibnerová. 1974. The inorganic origin of "anellotubulates." Micropaleontology 20(l):97-102; (b) Merek, E. L. 1973. Imaging and life detection. BioScience 23(3):153-159; (c) Gutstadt, A. M. 1975. Pseudo- and dubiofossils from the Newland Limestone (Belt Supergroup, late Precambrian), Montana. Journal of Sedimentary Petrology 45(2):405-414.
[21]Schopf and Packer (see Note 16).
[22]Cowen, R. 1990. History of life. Blackwell Scientific Publications, Boston, Oxford and London.
[23]Buick, R. 1990. Microfossil recognition in Archean rocks: an appraisal of spheroids and filaments from a 3500 m.y. old chert-barite unit at North Pole, Western Australia. Palaios 5:441-459.
[24]Ginsburg, R. N. 1991. Controversies about stromatolites: vices and virtues. In D. W. Müller, J. A. McKenzie, and H. Weissert (eds.), Controversies in Modern Geology, pp. 25-36.
[25]Hoffman, P. 1973. Recent and ancient algal stromatolites: seventy years of pedagogic cross-pollination. In R. N. Ginsburg (ed.), Evolving Concepts in Sedimentology, pp. 178-191. The Johns Hopkins University Studies in Geology No. 21, Baltimore and London.
[26](a) Thomas, C. 1968. Vadose pisolites in the Guadalupe and Apache Mountains, West Texas. In B. A. Silver (ed.), Guadalupian Facies, Apache Mountains Area, West Texas. Symposium and Guidebook 1968 Field Trip, Permian Basin Section, Society of Economic Paleontologists and Mineralogists Publication 68-11; (b) Estaban, M. and L. C. Pray. 1975. Subaqueous, syndepositional growth of in-place pisolite, Capitan Reef Complex (Permian), Guadalupe Mountains, New Mexico and West Texas. Geological Society of America Abstracts with Programs 7:1068-1069.
[27]Gutstadt (see Note 20).
[28](a) Monty, C. L. V. 1986. Range and significance of cavity-dwelling on endostromatolites. Sediments Down-Under. Abstracts of the 12th International Sedimentological Congress, Canberra, Australia, p. 216; (b) Vachard, D. and S. Razgallah. 1988. Survie des genres Tharama et Renalcis (Epiphytales, a1gues problématiques) dans le Permien supérieur du Djebel Tebaga (Tunisie). Comptes Rendus De L'Académie des Sciences Paris 306(Series II):1137-1140.
[29](a) Knoll, A. H. 1990. Precambrian evolution of prokaryotes and protists. In D. E. G. Briggs and P. R. Crowther (eds.), Palaeobiology: A Synthesis, pp. 9-16. Blackwell Scientific Publications, Oxford and London; (b) Woese, C. R. 1987. Bacterial evolution. Microbiological Reviews 51(2):221-271.
[30]Nagy, B., M. H. Engel, J. E. Zumberge, H. Ogino, and S. Y. Chang. Amino acids and hydrocarbons ~3,800-Myr old in the Isua Rocks, southwestern Greenland. Nature 289:53-56.
[31]Knoll, A. H. 1992. The early evolution of Eukaryotes: a geological perspective. Science 256:622-627.
[32]Snelling, A. 1991. Creationist geology: where do the 'Precambrian' strata fit? Creation Ex Nihilo Technical Journal 5(2):154-175.
[33]Wise, K. 1992. Some thoughts on the Precambrian fossil record. Creation Ex Nihilo Technical Journal 6(l):67-71.
[34]Clark, H. W. 1946. The new diluvialism. Science Publications, Angwin, California.
[35]Stewart, p. 31 (see Note 17).
[36]Schopf, J. W. 1968. Microflora of the Bitter Springs Formation, Late Precambrian, central Australia. Journal of Paleontology 42:651-688.
[37]Knoll 1990, p. 15 (see Note 29).