©Copyright 2018 GEOSCIENCE RESEARCH INSTITUTE
11060 Campus Street • Loma Linda, California 92350 • 909-558-4548

CHEMICAL EVOLUTION
by
Rene Evard
Associate Professor of Biochemistry,
School of Medicine,
Loma Linda University
and
David Schrodetzki
Senior,
Department of Chemistry,
Loma Linda University
“Le monde m’embarrasse, et je ne puis pas songer que cette horloge existe et n’a pas d’Horloger”
“Nature embarrasses me, and I cannot fathom that this clockwork exists while there is no clock maker.”
Voltaire .
INTRODUCTION TO THE PROBLEM
The chemical investigations that have developed from efforts to support the ideas set forth in Darwin’s The Origin of Species [1] have given rise to a biochemical hypothesis which attempts to explain the origin of life as an evolutionary progression from simple prebiotic molecules to the complex and integrate biomolecules of today’s living organisms. Whether these organisms are as complex as man or as simple as an amoeba, the biochemical evolutionist assumes that both ultimately arose by the transformation of simple molecules into an exceedingly intricate living system. Darwin’s theory that phylogeny has increased in complexity over immense periods of time [1] has gained pervasive acceptance. This has produced efforts to demonstrate experimentally that biological compounds could have been formed under prebiotic conditions. Such efforts are based on the assumption that life emerged spontaneously on the surface of the primitive earth after normal chemical processes had brought carbon-containing molecules to a stage of complexity that would make a living organism possible.
The first comprehensive treatments of biochemical evolution were published early in this century by A. I. Oparin [2], [4], [5] and J. B. S. Haldane. [3] The Oparin-Haldane hypothesis centers around the transformation of single atoms into complex precursors of living systems by means of an intense energy source such as solar ultraviolet radiation or lightning (electrical discharge) in a reducing atmosphere. Such an atmosphere would have been composed of some of the hydrides of elements in the 2nd and 3rd periods of the periodic chart: water (H2O), ammonia (NH3), methane (CH4), hydrogen sulfide (H2S), as well as free hydrogen (H2). Furthermore, Oparin and Haldane presumed that the nonvolatile precursors diffused into a primitive sea which served as the medium for the transformation of simpler reduced compounds of carbon and other elements into polypeptides and polynucleotides. In their model droplets that had accumulated various organic compounds eventually formed, causing intrasequential reactions spawning a primitive type of natural selection. Only those droplets which could stockpile the raw materials essential for self-perpetuation were allowed to survive. [4] Thus over a period of eons primordial micro-organisms containing many of the biochemical pathways fundamental to life began to flourish.
Direct experimental evidence seeming to validate the Oparin-Haldane hypothesis was first produced in 1953 by S. L. Miller. [6] This led to many other laboratory investigations of the prebiotic precursors that are thought to have occurred on a primitive earth. Based ultimately on the Oparin-Haldane hypothesis, these experiments have served as models depicting the events that are now speculated to have led to the origin of life.
The complex organization of a primordial organism, one which has acquired the most minimal requirements for life, necessitates a wide variety of proteins and nucleic acids. Furthermore, a model for prebiotic formation of these components must be consistent with current geological, biochemical, and astronomical theories.
Before attempting a discussion of experiments dealing with chemical evolution, a brief introduction to some basic biochemical concepts will be helpful. The study of living systems can be divided into descriptive and dynamic aspects: the chemical components themselves, and the reactions taking place in the living cell.
Some of the chemical elements appear to be more “fit” for life: only 27 of the 90 natural chemical elements are essential to living systems. Four elements (carbon, nitrogen, oxygen and hydrogen) make up most of the mass of living cells. All biomolecules, in turn, can be derived from simpler low molecular weight precursors: water, carbon dioxide, atmospheric nitrogen, and possibly ammonia. These precursors can be converted by living cells into larger biomolecules such as amino acids, simple sugars, purines, pyrimidines, glycerol, and fatty acids which, when linked to each other, form the macromolecules of the cell. Thus, proteins are made up of 20 different amino acids linked together. The mononucleotides (made up of either purines or pyrimidine bases, simple sugars, and phosphate) combine to form the nucleic acid. Both proteins and nucleic acids are large biomolecules with molecular weights ranging from about 10,000 to millions.
The next level of organization includes supramolecular structures involving inner cell membranes and complex organelles such as the nucleus, the mitochondria and the ribosomes. Thus a living cell is made up of a wide range of specific compounds as well as highly organized subcellular structural components working together to carry the functions associated with life.
The dynamic aspect is the study of the many reactions taking place simultaneously in a living cell, allowing it to utilize energy in order to grow, develop, differentiate and reproduce. Because these processes all require the continual synthesis and breakdown of a large number of complex chemical entities, they must be under strict control and regulation in order to maintain the normal operation of life within the cell. A living cell is much more than a mixture of chemical compounds placed at random into a small bag; rather, the simplest cell is a highly specific and organized entity possessing tremendous chemical and biological capabilities.
Proteins perform a large number of functions within biological systems, the nature of which depends upon the number and order (sequence) of the amino acids within the molecules. The order is critical. In some instances, having one amino acid out of position will cause a protein to be non-functional. Proteins act as enzymes, which are catalysts involved in all biological reactions; they may serve for storage as a source of amino acids; some are also hormones (messengers) regulating the rate of certain reactions and transmitting messages from one organ to another. All these functions depend upon a specific arrangement of the component amino acids. In addition to chemical functions proteins are an important part of the physical framework of cells and tissues.
Since proteins and nucleic acids make up the most important components of cells, both in terms of function and bulk composition, we shall focus our attention on the experiments dealing with attempts to produce these in the laboratory under presumed prebiotic conditions.
THE CONDITION OF THE PRIMITIVE ATMOSPHERE
The assumption that the earth’s primitive atmosphere predominately contained large amounts of hydrogen (i.e., a reducing environment) is primarily a matter of conjecture.
S. L. Miller in his recent publication7 states:
Arguments concerning the composition of the primitive atmosphere are particularly controversial. It is important, therefore, to state our own prejudice clearly. We believe that there must have been a period when the earth’s atmosphere was reducing, because the synthesis of compounds of biological interest takes place only under reducing conditions.
Under the influence of an intense energy source the reduced gases (i.e. H2S, H2, CH4, NH3, N2, and H2O) are thought to have evolved into the primordial precursors which would result in the development of a living organism. Some indirect evidence does seem to validate such a theory.
When hydrogen was discovered to be the most abundant element in our solar system, it seemed most reasonable to conclude that, “as the Earth was forming, most of its carbon, nitrogen, and oxygen would be in the form of methane, ammonia, and water.” [8] However, in the light of current geological and geophysical data, it appears that ammonia on the primitive earth would have been quickly destroyed by ultraviolet radiation. [9] Furthermore, if large amounts of methane had ever been present in the earth’s atmosphere, geological evidence for this should also be available. Laboratory experiments show that one consequence of irradiating a dense, highly reducing atmosphere is the production of hydrophobic organic molecules which would be absorbed by sedimentary clays. Consequently, the earliest rocks should contain an unusually large proportion of carbon or organic chemicals. This is not the case. [9]
Abelson [9] and Cloud [10] further state that the primitive atmosphere may have been an oxidizing environment. In other words, the elements of the primitive atmosphere had combined with oxygen as it occurs today. Such an atmosphere would contain oxidized compounds as CO2, H2O, N2, O2, and SO2. However, argumentation for a reducing environment continues relentlessly as scientists today point out that oxygen has a deleterious effect on many aspects of metabolism, because most organic compounds decompose in the presence of free oxygen. The presence of Fe (ferrous iron) in the earlier part of the geological record provides further evidence for a reducing atmosphere. Because ferrous iron is unstable in the presence of O2, it is thought to have existed in an oxygen-free environment. [11] However, even Miller [7] notes that this does not prove a reducing atmosphere. Additional evidence that a reducing atmosphere may not have been present has been given in a previous issue of this journal (see Origins 2:59-63).
The arguments for and against a primitive reducing atmosphere may never be adequately resolved. Our view is that scientists may be attempting to fit data into a predetermined mold (i.e. a reducing atmosphere). Arbitrary definition of a system, such as a reducing environment, sets limits to scientific investigation which then becomes bound to the rules of the assumed system. This is not science in its most empirical form.
The most significant source of energy for our planet today, and on a prebiotic earth, is the sun. This solar energy includes ultraviolet radiation and is complemented by lightning (electrical discharge). Laboratory experiments are fashioned primarily around these sources. Other possible sources include volcanoes, shock waves, and radioactive as well as cosmic rays. Thus simulation of a presupposed primitive atmosphere in a given laboratory can become quite involved. Electrical sparks and corona discharges (simulating lightning) as well as x-rays and electron beams (simulating cosmic rays and radioactivity in rock) and heat (simulating the thermal effects of volcanoes), are but a few of the techniques applied to the synthesis of precursors to life as we know it today.
AMINO ACID SYNTHESIS
Biochemical evolution assumes that laboratory experiments can be used to duplicate primitive-earth events. [3], [5] According to Kenyon & Stein- man, [12] such experiments may have two possible implications: a) because many approaches result in the same significant products, biochemical evolution took place under several different environments, all of which contributed to the same end, or b) these experiments are mostly only a demonstration of interesting chemical phenomena.
With this latter view a possibility, the biochemical evolutionists must consider carefully data concerning the plausibility of an environment which seems to allow for the transformation of nonliving material into life. Should the evolutionist neglect relevant findings which mitigate against such an environment, one should be skeptical of his conclusions. Again, the proposal that there was a reducing atmosphere on a primitive earth, though not entirely without foundation, has serious problems. As such, one cannot be totally certain that the inferences drawn from data based on this assumption have any bearing on the actual course of chemical evolution.
Assuming that the primitive atmosphere could have been reducing, let us consider the first experiment done in such an environment and carefully review the results. Miller [6], [43] working at the University of Chicago in 1953, put the components of a reducing atmosphere (ammonia, methane, hydrogen and water) within an apparatus with a high energy source, in this case, an electrical discharge. This energy source was used since it simulates lightning, which is thought to be an important source of energy on a prebiotic earth. Note the apparatus in Figure 1.
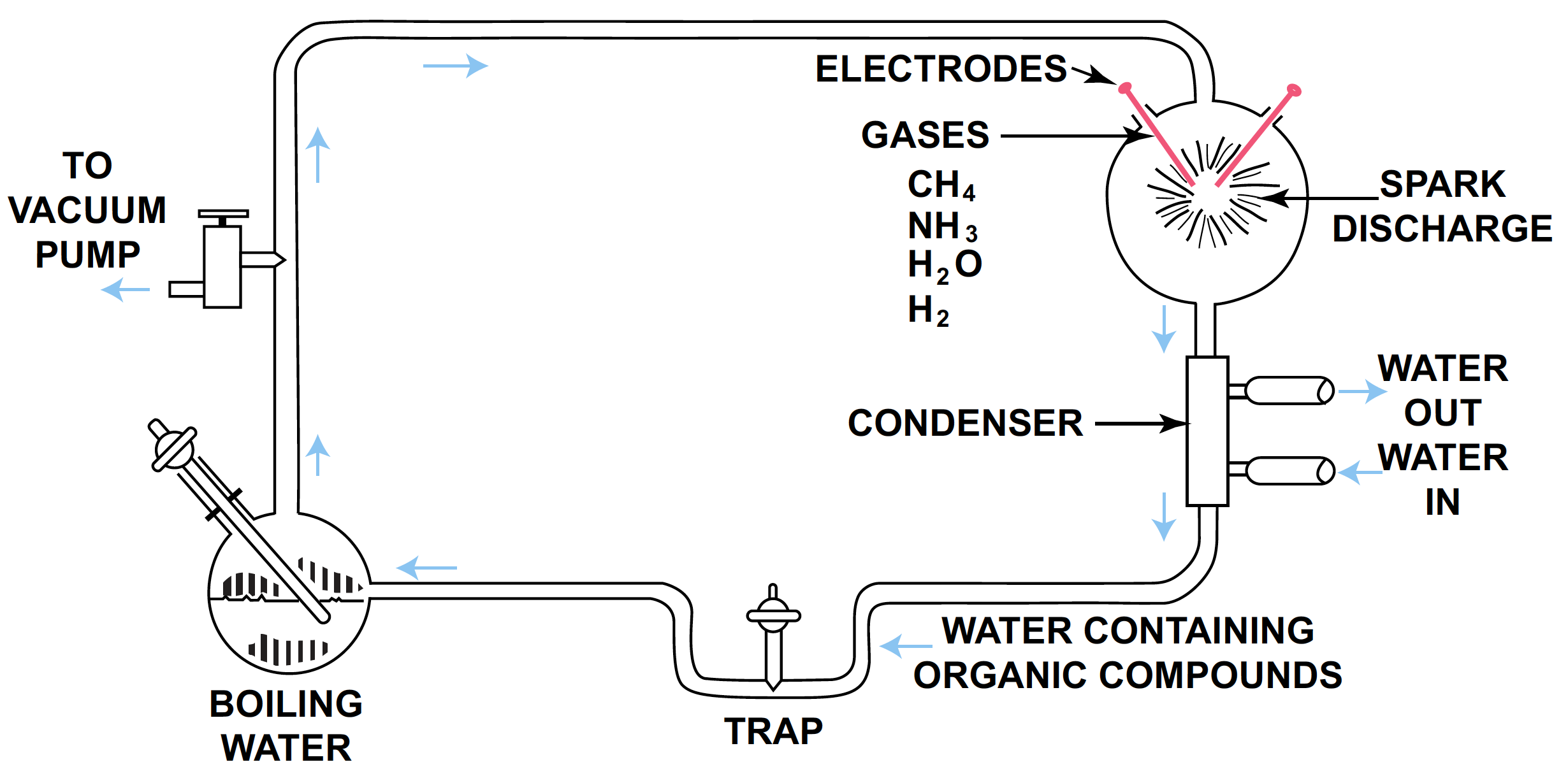
FIGURE 1. S. L. Miller’s apparatus used in his classic experiment. [16]
The gaseous mixture was admitted to the apparatus and caused to circulate in a clockwise direction when water was heated in the lower sphere. The mixture passed through the electrodes and was liquefied within a condenser below the sparking chamber. The products formed were then washed down into water and captured within a trap.
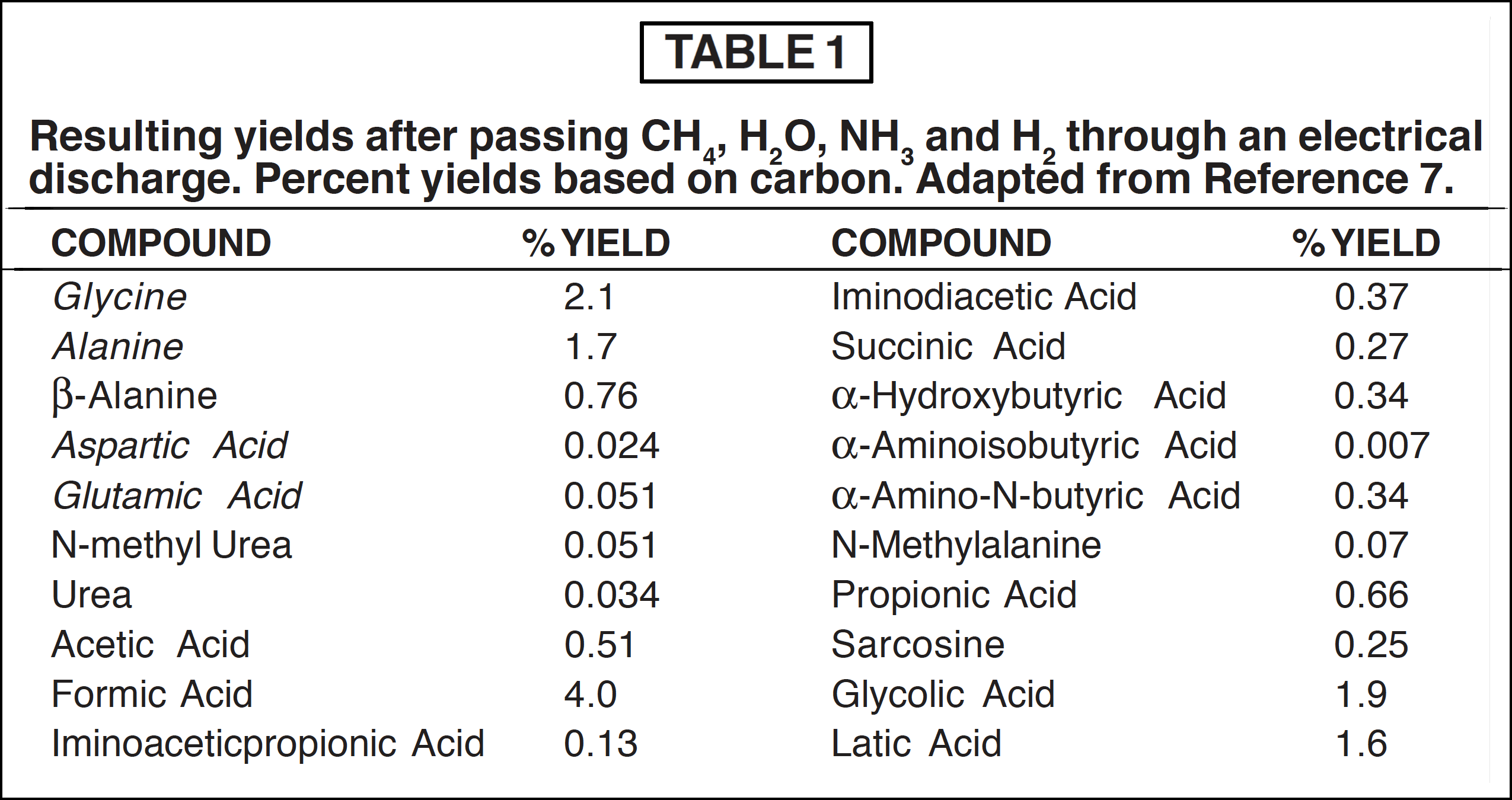
After one week of sparking, the products were removed from the trap and analyzed by anion-cation-exchange chromatography. The products and yields are summarized in Table 1. The important amino acid precursors have been underlined.
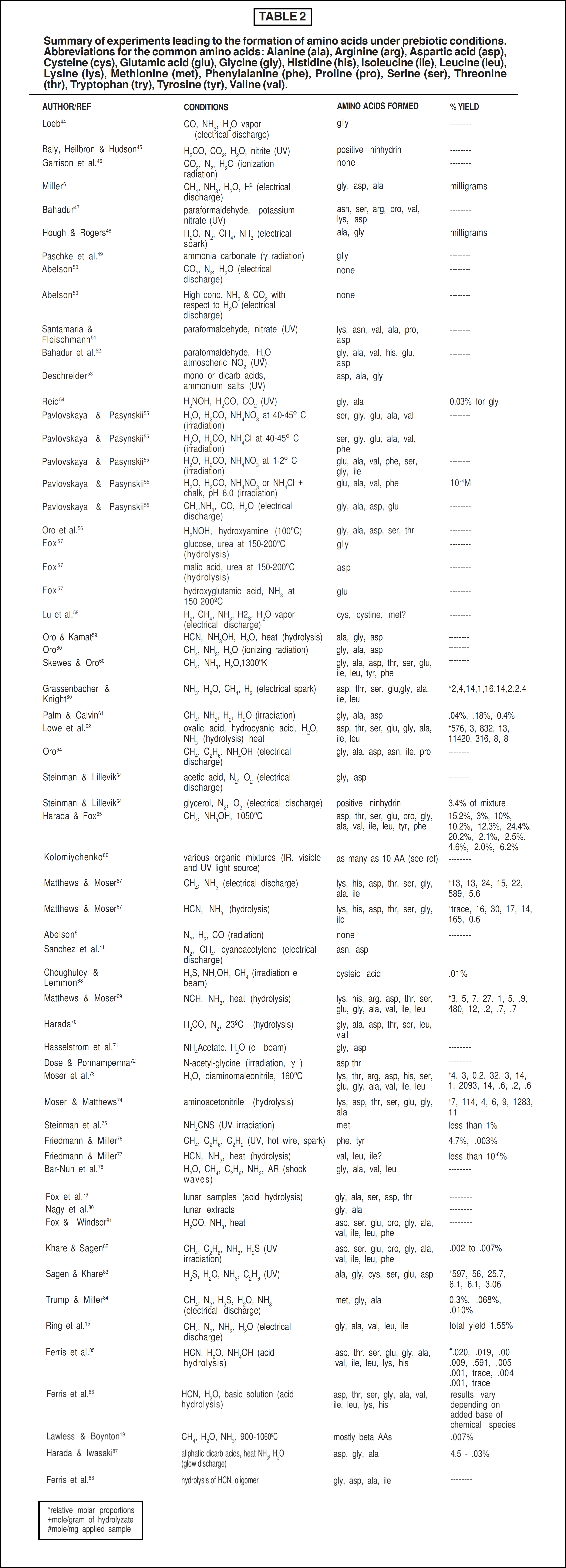
Since Miller’s classic work, several variations of his experiment have been carried out by other researchers using basically the same type of apparatus. Table 2 provides a list of the men and their work, with the analytical results of their products.
PROBLEMS WITH AMINO ACID SYNTHESIS
At present, 18 out of the 20 amino acids found in proteins have been synthesized by methods similar to Miller’s classic experiment. Tryptophan and glutamine have not been identified among the reaction products. Interestingly enough, two amino acids, tyrosine and phenylalanine, have been produced only on heating mixtures of the presumed prebiotic gases to over 1000oC. [8] These results are not consistent with the overall evolutionary hypothesis which says that the synthesis must have taken place at temperatures less than 150oC. [13], [14] Also, most amino acids are especially susceptible to decomposition by irreversible decarboxylation caused by heat. [7]
Richard Lemmon [8] notes that there is an intrinsic need for controls to eliminate the presence of bacteria and other contamination in experiments dealing with the synthesis of amino acids. This point seems very valid, for amino acids present within various types of bacteria caught in the experimental apparatus may be picked up by any number of methods used in identifying amino acids. Notwithstanding, the only reliable methods em- ployed for the identification of amino acids to date are the mixed melting- point derivatives or an analysis by gas chromatography and by a mass spectrometry. [15] As Miller [15] states:
The correct elution time on the amino acid analyzer is insufficient by itself to identify an amino acid. Many amino acids not found in proteins have peaks that coincide with protein amino acids. These same limiting factors are true for gas chromatography, or electrophoresis, even with different solvents.
This information gives some grasp of the difficulties encountered and the reliability of amino acid identification.
Turning to the problems of the actual synthesis of amino acids, one must note the thermodynamic stability of the products formed in the reducing atmosphere that produced them. Simply stated, the reactions that create the amino acids also tend to destroy them. [9], [16] This is due in part to the strength of the energy source. One feature of Miller’s apparatus and subsequent variations of his experiment is a trap suitable for the storage and/or the immediate removal of the products of the reaction. [12] Thus, one must propose the existence of a primitive trap [24] on earth during the early phases of the chemical evolutionary process. Without such, the destructive forces of electrical discharges or ultraviolet radiation would destroy the prebiotic precursors of life that they had produced. A primitive-earth trap has been suggested by Bernal; [17] however, it seems precluded by Hull. [18]
Considering the thermodynamics of chemical evolution, especially the equilibrium concentrations of synthesized organic compounds, Hull demonstrated that the accumulation of amino acids on a primitive earth would result in a concentration hopelessly low and totally unsuitable as a starting material. [18] Calculating not only the relative rates of formation of several amino acids, but also the rates of their decomposition, Hull found the resultant concentrations to be on the order of 10-12 moles/liter or less. For instance, in calculations concerning glycine, the simplest amino acid, the mean concentration would be between 10-12 and 10-27 m/l, far below the 10-2 molar concentrations thought to be necessary for the chemical evolutionary development of life. [8] As other organic compounds are considered, the concentrations become even smaller (glucose 10-134 m/l). [18] Quoting Hull:
The conclusion from the arguments presents the most serious obstacle.... First, thermodynamic calculations predict vanishingly small concentrations of even the simplest organic compounds. Secondly, the reactions that are involved to synthesize such compounds are seen to be much more effective in decomposing them. [18]
The yields of key amino acids such as aspartic and glutamic acids (Table 2) are very low and not at all in proportion to the biological system concentrations. The total yield of these two compounds is less than 0.07%, while other important amino acids are not even present under the conditions producing these. One should keep these particular amino acids in mind, because they are very significant when one speculates on mechanisms for the polymerization of amino acids into polypeptides.
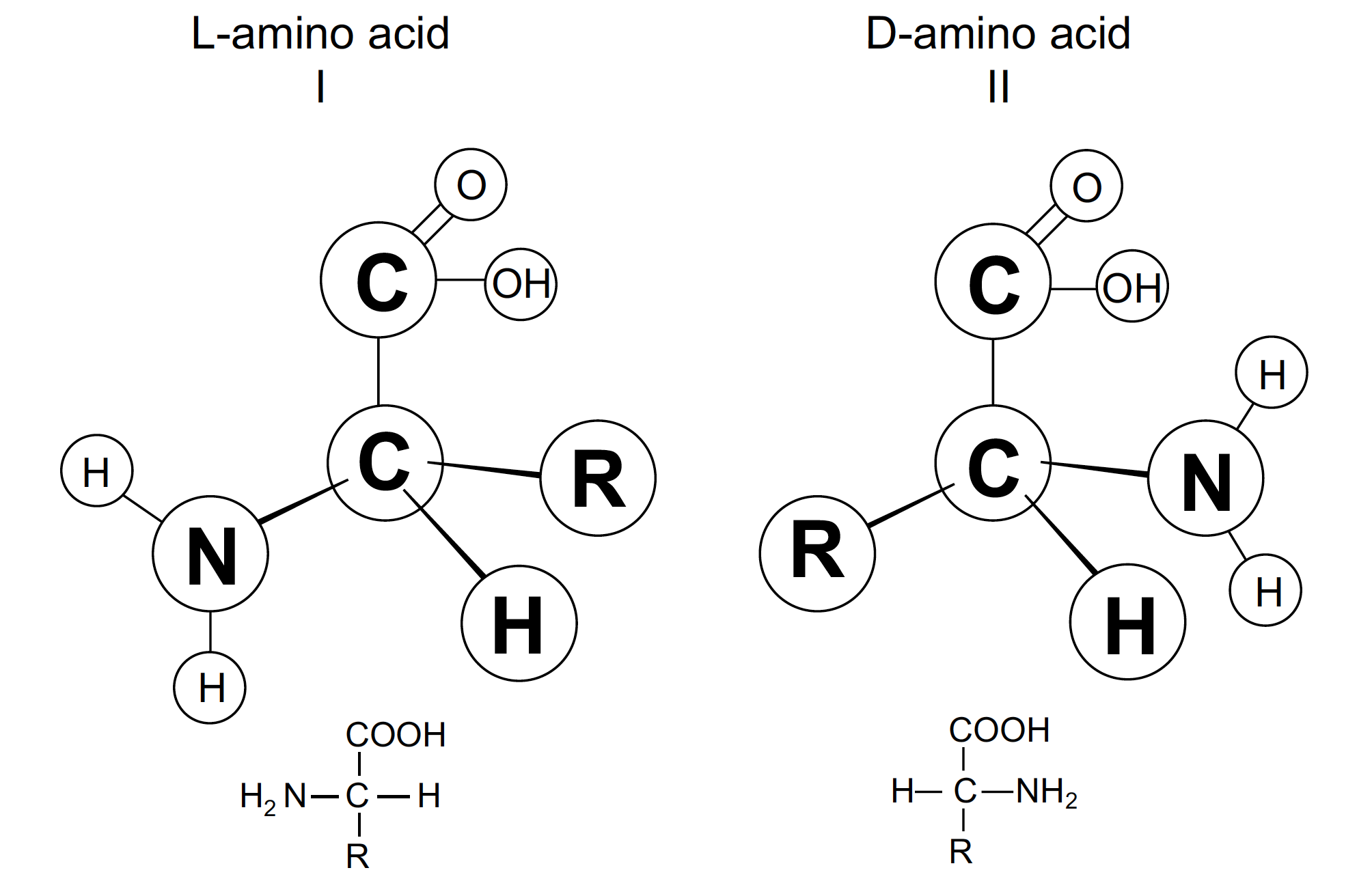
FIGURE 2. Optical isomers (D- and L-forms) of an amino acid. Note that one form is a mirror image of the other.
STEREOCHEMISTRY
Stereochemistry deals with the three-dimensional structure of chemical compounds. Nearly all organic molecules, especially the amino acids and sugars, may exist in more than one three-dimensional arrangement. For instance, amino acids found in proteins have the amino group located at the a-carbon (next to the carboxyl group) on the carbon skeleton. While biological systems do utilize amino acids that have other types of structure, only the alpha type are found in proteins.
In addition, alpha amino acids themselves may exist in two different forms: the D- and the L-configurations. These are called optical isomers (see Figure 2). Optical isomers have the same relationship as the right and left hand of a person. Possessing identical chemical properties, they differ in one physical property: the behavior under the influence of polarized light. Proteins fundamental to the maintenance of living systems are composed exclusively of L-amino acids. This configuration is required in order for the particular protein molecule to attain the shape that is critical for its biological function.
A principle of organic chemical synthesis is that reactions starting with an optically inactive mixture of reactants will yield an optically inactive product. This is the case when amino acids are synthesized from simple organic molecules. The amino acids formed are a mixture of equal amounts of the D- and L-forms, yet biological systems have the unique ability to incorporate only the L-form into proteins and completely exclude the D- form. This critical point in the problem of chemical evolution has not been resolved satisfactorily.
Amino acids produced under prebiotic conditions designated would more than likely contain equal parts of the D- and L-isomers (a racemic mixture). [12] As noted, those amino acids found in living systems are of the L-α-configuration. Thus any hypothesis dealing with chemical evolution must ultimately account for the incorporation of the specific L-α- configuration over the other alternatives. Indeed, there have been many attempts to account for the origin of such specific optically active compounds within living organisms (see Table 3).
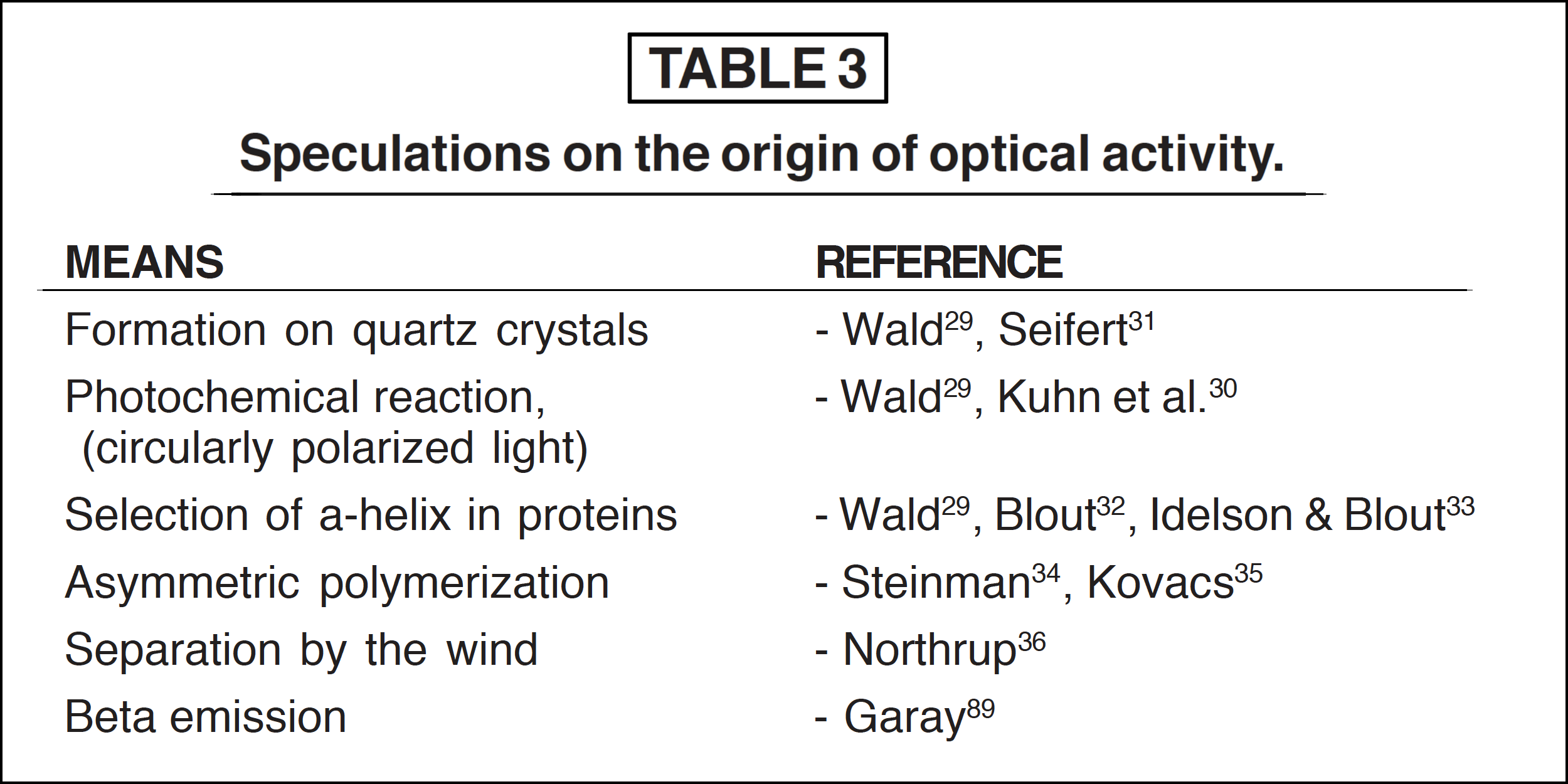
The proposal that the stereo homogenity of the biologically active amino acids has come about through polymerization on the surface of optically active quartz is no longer accepted. [29], [31] When quartz is used to orient the amino acids into specific configurations, D- and L-amino acids are selected to the same degree. [31] As for the possibility of the stereochemical phenomenon occurring from circular polarized light and the resultant reactions, very little rotation within the acids is found. [12], [29], [30] Furthermore, this assumption is not sound on theoretical grounds, because D-amino acids do not necessarily rotate the plane of polarized light to the right, and L-amino acids do not always rotate the plane to the left. Therefore, if circular polarized light was used to induce asymmetric synthesis, it would produce both D- and L-amino acids.
The selection of L-α-conformation did not occur as a result of the specificity of the α-helix for L-amino acids, as it has been found that limited regions of an α-helix of either polarity could be formed in a racemic mixture of amino acids. [7], [12], [29], [32], [33] Worthy of note also is the fact that asymmetric polymerization has been found to produce a certain degree of racemization among amino acids. [12], [34], [35] Finally, Northrup’s proposal [36] that separation could have occurred by the natural forces of the wind, after the drying of a mixture of D- and, L-amino acids, seems totally preposterous.
Experiments performed by Lawless [19] yielded a predominance of the beta-amino acids, rather than the alpha form. He suggests:
The formation of a primitive organism in an environment requiring the utilization of beta-amino acids, followed by the evolution of an organism that utilizes alpha amino acids is...unattractive.
All biological systems have the unique ability to differentiate between stereoisomers. This unique stereochemistry is required at the molecular level so that larger molecules will have the proper shape allowing them to carry out their varied and specific functions within the living cell. This shape is again important in determining the activity and the proper functioning of subcellular structures of the cell. There is a definite order and organization associated with living systems, and the stereochemistry of the basic building blocks is one of the key components of this beautiful structure.
POLYMERIZATION
Polymerization is the joining of molecular subunits which form protein, nucleic acids and other complex molecules in biological systems. Such a process not only involves the formation of chemical bonds and the elimination of water, but the specific sequence or linear arrangement of the subunits is what causes these molecules to be biologically active. The specific activity of each biochemical reaction is due to the specific arrangement of amino acids in proteins, or nucleotides in nucleic acid (the backbone of the gene structures). The displacement of a single amino acid or nucleotide may alter the biochemical function of a polymer. This alteration may be so crucial that its ultimate effect could be death to the organism.
The polymerization of biomolecules involves reversing a thermodynamic barrier, an energy barrier which does not allow monomers (the molecular subunits) to spontaneously combine to form polymers unless they have been activated or energy is supplied.
Several mechanisms for such polymerization have been proposed. After Hull stated that the concentration of the prebiotic precursors in the oceans would never have reached an appreciable level for self-polymerization, researchers have sought other devices. One envisioned by S. W. Fox and others [20], [21], [22] involves the use of a dry, pure mixture of amino acids and high concentrations of glutamic and aspartic acids, while employing thermal (heat) activation as an energy source. Heating the mixture at 175oC for 2 to 3 hours converts about 13% of it to a water-soluble polymer, made up of many kinds of amino acids. [22] When dissolved in hot water and allowed to cool, the polymer precipitates, forming spherical globules said by Fox to resemble coccoid bacteria, the so-called “proteinoids.” [23], [24]
Such may be the case; however, one must note: a) if this mixture were heated for more than several hours, the polymers would have been destroyed (on a prebiotic earth the mixture would have been heated for a considerable length of time, and thus easily destroyed), and b) high concentrations of glutamic and aspartic acids were used, while the results of experiments dealing with the synthesis of these acids yielded less than 0.07%. In reference to a) cited above, it is also difficult to conceive of a primitive-earth environment that would allow a mixture of amino acids, high in purity, dry, rich in glutamic and aspartic acid, to react at 175oC for no more than 6 hours, then cool, allowing for polymerization.
To compound the problem, the polymerization of amino acids by heating shows a marked degree of racemization of the optically active starting reagents,20 and stereoselective catalysts and surfaces would be nonexistent on a prebiotic earth. [12]
Investigators have discovered several means of enhancing the yields of many polymerization reactions through the use of acids, a process known as chemical activation. The presence of phosphoric or polyphosphoric acid nearly doubles the typical yield. [20], [25], [26] Also, it has been demonstrated that peptide bonds between amino acids may be promoted by cyanamides in acidic solutions. [27], [28] While these facts seem to present a more realistic solution in terms of increasing the yield in a primitive ocean, these compounds are either acidic themselves or in acidic solution. As such the primitive pH of the ocean, calculated to be 8.0-8.1,7 would be lowered, thus making the seas an environment unsuited for chemical evolution. Since many organic compounds are unstable and dissociate below a pH of 7, it is doubtful that the addition of acid solution naturally would enhance the chance of survival of a primitive organism should it have evolved. Similarly, histidine (an amino acid) is found to be relatively unstable, particularly to acid hydrolysis. [7] Another unattractive feature is that polymerization with dilute cynanamide solutions yields short poly- peptides. [12] No mechanism is yet available to explain the synthesis of larger molecules, except by saying that, “in time,” such a phenomenon could have occurred.
Because the sequence specificity in proteins is so important, one must ask how such could have arisen abiologically, and if so, by what processes and constraints. As the number and different kinds of amino acids within a single polymer increase, so does the number of possible sequence structures16 (see Table 4).
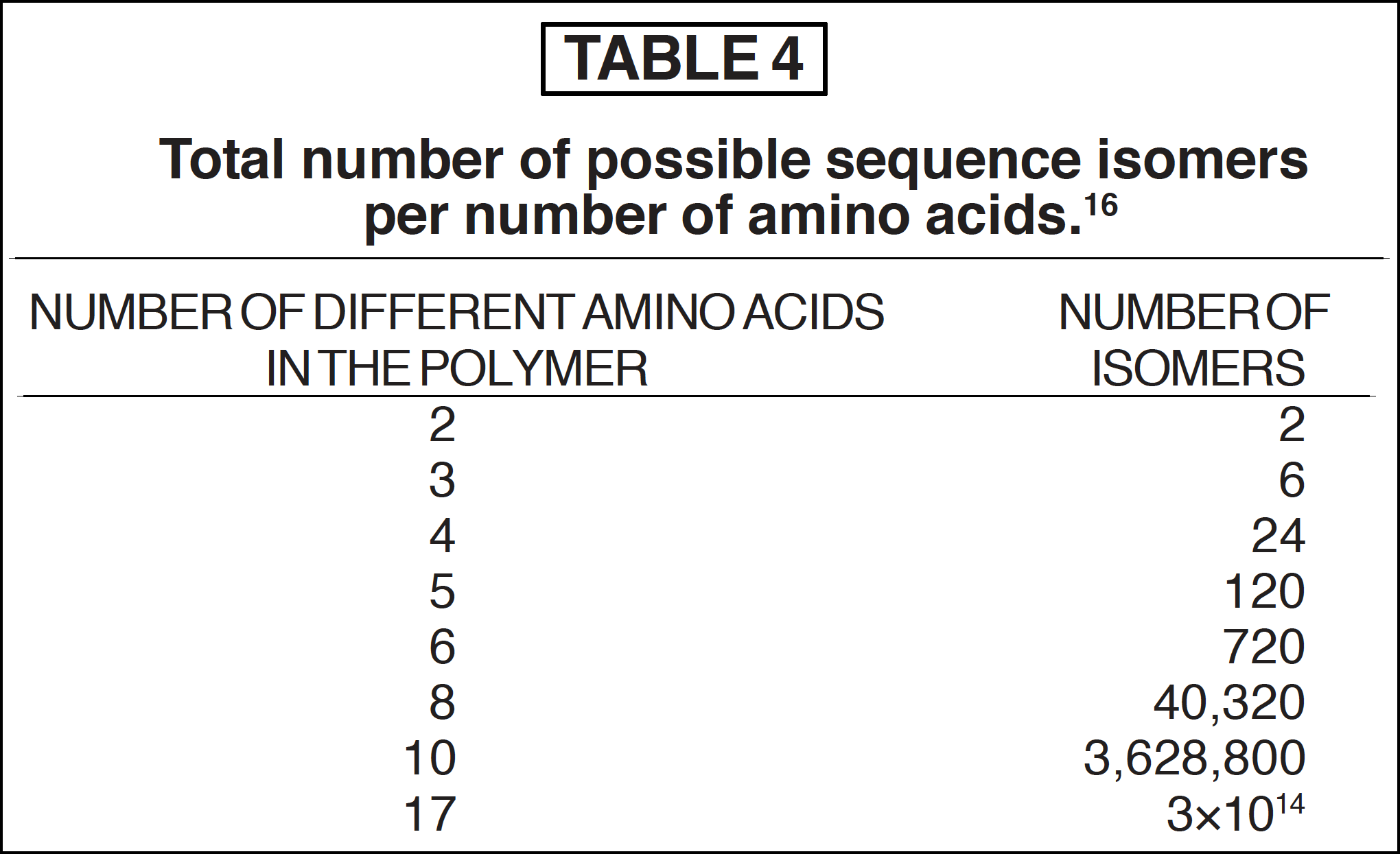
Recall that a polypeptide can exist in any combination of 20 different kinds of L-α-amino acids. Should the formation of polymers have been a random event, the existence of a single molecule of every possible sequence of a polymer (isomers) with only 12 amino acid kinds would result in a total mass that would equal 10280 grams (1 followed by 280 zeros) [37] (see Table 5). Hence the chance of obtaining a specific polymer by random events seems hopelessly low.
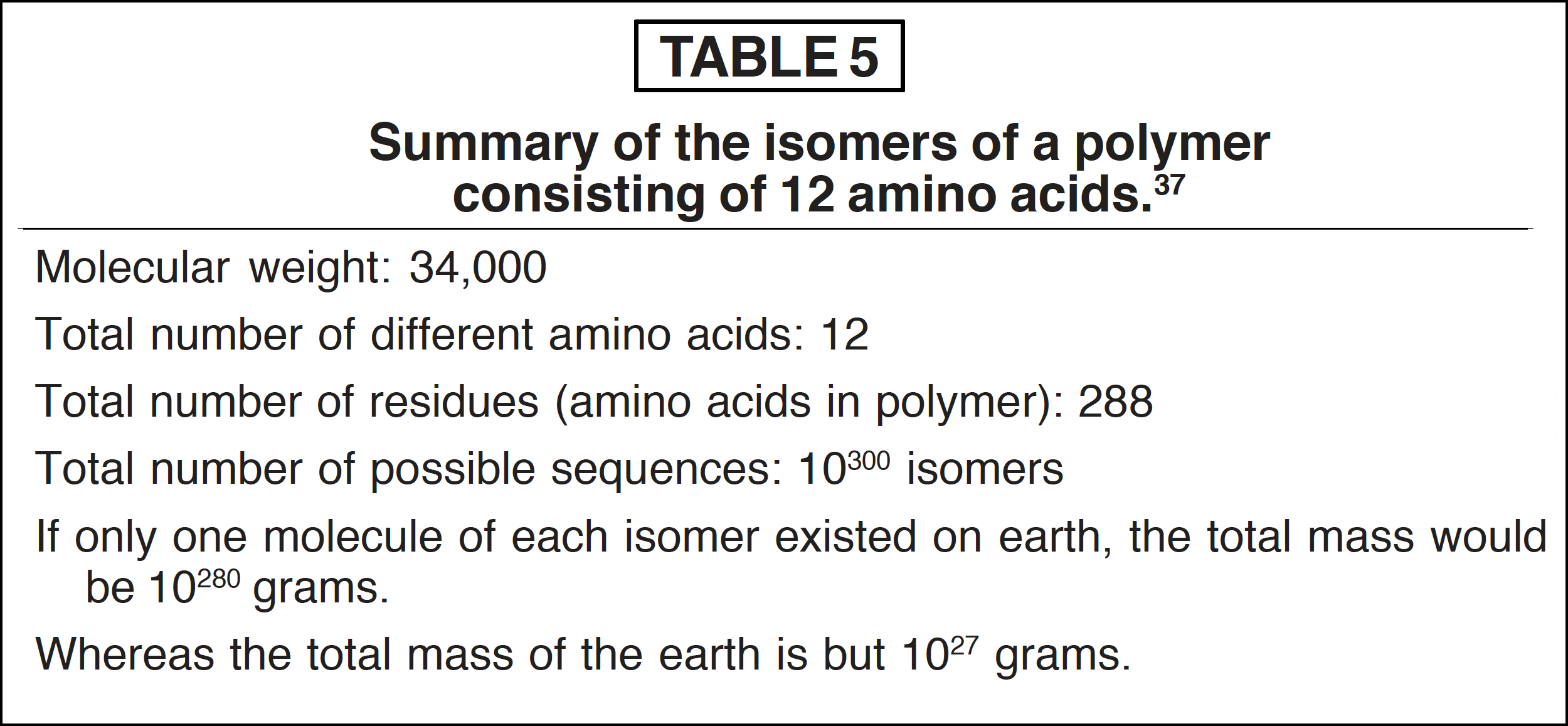
In view of this consideration, the firm establishment of a mechanism for the polymerization of the amino acids produced under presupposed abiotic conditions is very difficult to formulate. Though such a process cannot be looked upon as totally impossible, it is clearly not an adequate basis for explaining the emergence of life as we know it today. Therefore we must seek new vistas of understanding, or perhaps the rebirth of more plausible ideas.
NUCLEIC ACID SYNTHESIS
Nucleic acids are another key component of biological systems. As we have mentioned previously, nucleic acids are made up of: a) five organic bases (adenine, guanine, uracil, cytosine, thyamine), b) two sugars (ribose or deoxyribose), and c) phosphoric acid (Figure 3). The basic unit of the nucleic acid is the nucleotide, a composite structure consisting of these three components. When properly linked in a sequence, the nucleotides form either deoxyribonucleic acid (DNA) or ribonucleic acid (RNA), depending on the nature of the sugar present.
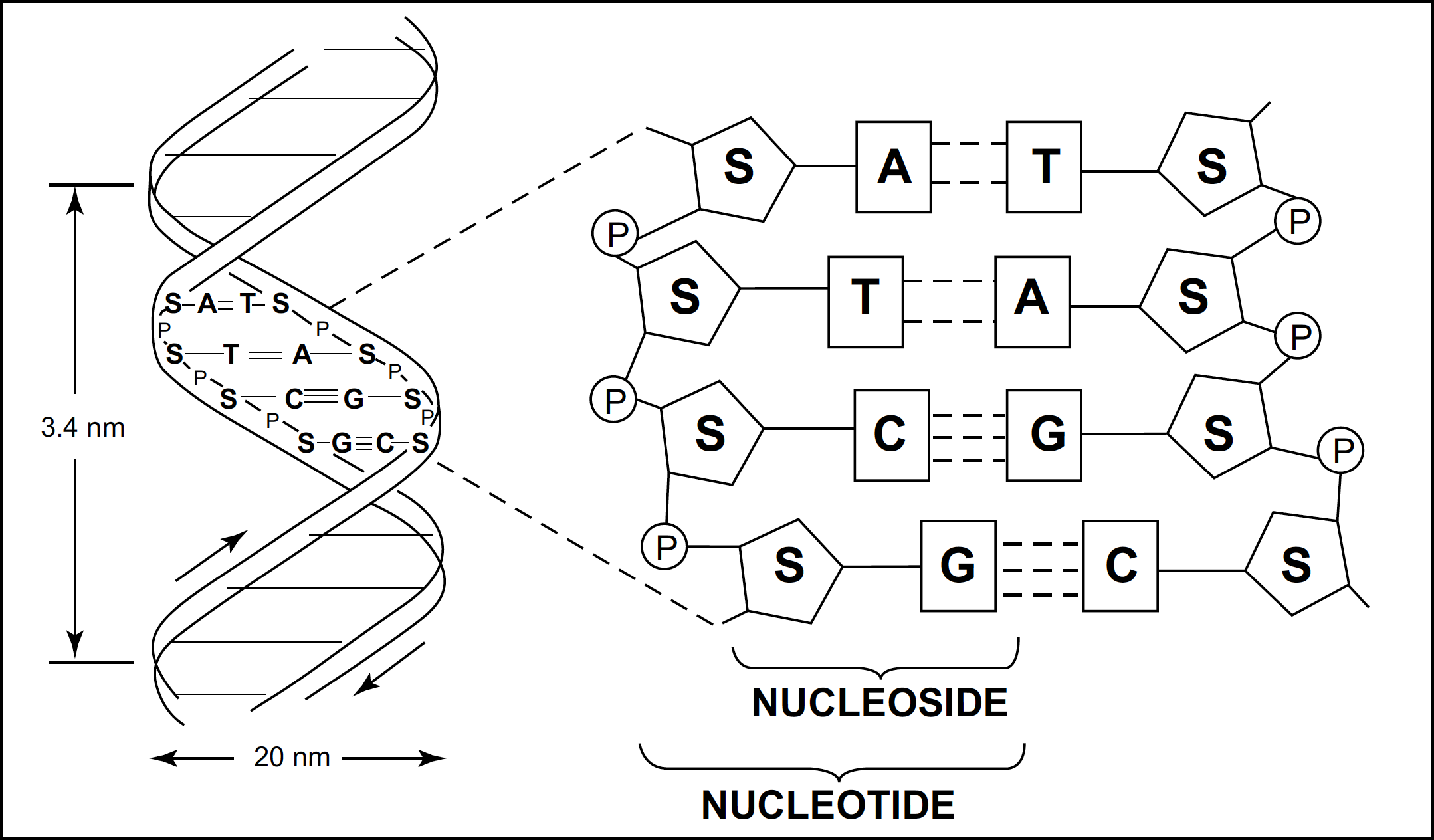
FIGURE 3. Schematic representation of the structure of DNA
A. The double helix of DNA.
B. The backbone of the two strands of DNA. A,T,G,C represent the bases adenine, thyamine, guanine, and cytosine respectively. S represents the sugar deoxyribose, and P is the phosphate. The two strands are joined through hydrogen bonding (dashed lines) formed between certain bases.
To produce nucleic acids, one must first account for the formation of their building blocks, the purines and pyrimidine bases, both ribose and deoxyribose sugars, as well as the incorporation of inorganic phosphate into these organic molecules.
Oro & Kimball synthesized a purine base, adenine, by condensing hydrogen cyanide in a concentrated ammonia solution (1-10 M). [38] However, the yield was extremely low (less than 5%), leaving one to speculate on how a concentration of adenine could be built up sufficiently to spontaneously coalesce with the other nucleotide components in a vast aqueous environment, the prebiotic sea. Also, the above experimental situation was rather drastic, since no one yet has explained the presence of such high concentrations of ammonia on a prebiotic earth. Orgel & Lohrmann undertook the study of a related synthesis of adenine in more dilute solutions, as they saw Oro’s work was not realistic in terms of reasonable prebiotic conditions. [42] Orgel states that if all the nitrogen in the atmosphere was converted to ammonium cyanide and dissolved in the oceans, the resulting solutions would not exceed 0.2 M in concentration. Because hydrogen cyanide is rapidly converted to formate, it is unlikely that the cyanide concentration in the oceans ever exceeded 10-4 M.42 To further complicate matters, hydrolysis of cyanide to foramide and formic acid becomes the dominant reaction for cyanide once its concentration drops below 0.01 M.7
Several researchers believe a more plausible explanation exists for the formation of concentrated cyanide, thus enabling the formation of adenine in appreciable yields. When the very dilute ammonium cyanide solution is cooled to temperatures below 0oC (-10 to -22oC), ice separates out, and a concentrated solution of NH4CH is obtained. At this eutectic temperature, the liquid phase contains about 75% hydrogen cyanide. In this way, excellent yields have been produced from dilute (0.001 M) cyanide solutions kept at -10oC.7, [50] However, Miller notes “that the presence of large amounts of salt greatly lowers the efficiency of cyanide polymerization in eutectics, since the eutectic volume is determined by the amount of salt present rather than cyanide.” [7] It is thought that such a synthesis and subsequent cyanide polymerization occurred on the frozen surfaces of lakes or oceans. This restricts the prebiotic milieu having the necessary conditions for the condensing of the nitrogen bases, sugars, and phosphate to form nucleotides.
Guanine, the other purine, has to be synthesized by the reactions of cyanate, urea or cyanogen. Its synthesis has not been studied in as much detail as that of adenine. [7]
As for the synthesis of pyrimidines, Ferris et al. have reported the production of uracil through a cytosine synthesis. [39] Cytosine hydrolyzes quite easily to uracil, one of the three pyrimidines. Cyanate and cyanoacetylene are also used to synthesize cytosine. [7] However, these compounds are highly unstable. Ferris also states that “the instability of cyanate and cyanoacetylene restricts severely the range of prebiotic environments in which such a synthesis could have occurred.” [39] Further complications arise because the half-life of cyanoacetylene is at most a few hundred years. Similarly, under any conditions, cyanate hydrolysis to ammonium carbonate takes place in less than one hundred years. [7], [40] Thus it is difficult to comprehend how the necessary high concentrations could have ac- cumulated in a primitive ocean. It is speculated that cyanate could have been concentrated somewhat during the evaporation of pools and then reacted with cyanoacetylene from the atmosphere. This is not very convincing, because cyanoacetylene is destroyed rapidly in the presence of ammonia, thus complicating the simultaneous synthesis of purines. [41]
SUGARS AND PHOSPHORYLATION
The prebiotic production of pentose sugars (part of the nucleotides) has also been investigated. In the mid-nineteenth century, Butlerow [7] observed that in strongly alkaline solutions, formaldehyde would condense, forming sugar-like molecules. Should sodium hydroxide, as the strong alkali, be mixed with formaldehyde, the Cannizzaro reaction occurs, but it does not give appreciable amounts of sugar.
There are problems with the synthesis of these sugars. As the reaction proceeds, the earliest product identifiable is glycolaldehyde, followed by glyceraldehyde, dihydroxyacetone, tetrose, pentose and hexose sugars including ribose. Yields must have been upwards of 50%; however, to get such yields, the reactions must be stopped completely at the appropriate moment. [7] The first question raised is how those sugars employed by the evolving system were preferred over the other sugars synthesized. This choosing process must be very specific, because a multitude of isomers would be found after such a synthesis. No answer to this problem has as yet been proposed. Unstable in aqueous solutions, especially above pH 7, sugars are destroyed under the conditions of the Butlerow reaction soon after they are formed. [7] Another difficulty is that the mixtures obtained by the sugars yield only a very small proportion of ribose. Finally, this reaction does not occur with formaldehyde concentrations below 0.01 M. Thus, one must again present an additional model showing that the formaldehyde concentration either rose above 0.01 M or that lower formaldehyde concentrations are capable of producing sugars in a primitive sea. [7]
Most workers in this field simply take for granted the actual synthesis of ribose under prebiotic conditions. Nevertheless this is a key component of the very fundamental nucleic acid molecule.
The third component of nucleic acids is phosphate. A number of mechanisms have been proposed for the phosphorylation of nucleosides. In one such experiment, the nucleoside uridine was heated with the inorganic phosphate Ca(H2PO4)2 at 65oC for nine months. [42] Uridine monophosphates along with small amounts of uridine diphosphates were produced. However, Ca(H2PO4)2 is precipitated only from acid solutions and it seems unlikely to have ever been a common mineral, especially in a reducing primitive environment. [48] One of the largest obstacles to overcome is that, at present, no experiments have been performed that satisfactorily show the incorporation of inorganic phosphate into an organic molecule under prebiotic conditions. [7] Nor has the next step (nucleotide polymerization) yet been positively demonstrated in the laboratory. In the words of Miller, “The origin of nucleosides and nucleotides remains...one of the major problems in prebiotic synthesis.” [7]
CONCLUSIONS
In this article we have attempted to critically analyze the results of laboratory experiments designed to demonstrate that life could have originated on this planet spontaneously.
While some data seems to support the hypothesis that the primitive atmosphere was reducing, evidence to the contrary must not be neglected. A problem in the deliberation over a reducing atmosphere compared to an oxidizing one lies in the way carbon appeared on the surface of the prebiotic earth. Should it have been outgased from the prebiotic earth as CH4, one would encounter a reducing milieu. If it was outgased as CO2, a potentially oxidizing atmosphere would be formed. It would seem that the only certainty as to the kind of environment that existed on a hypothetical primitive earth remains with the prejudice of the individual investigators.
Are there positive results from the standpoint of biochemical evolution?
Eighteen out of twenty amino acids have been produced under what is believed to have been the prebiotic conditions of the earth. Under specific conditions, researchers have also found that protein-like substances, the so-called “proteinoids,” have been produced. Likewise, four or five bases of nucleic acids have been synthesized, though phosphorylation of these components has been very difficult.
In Table 2, we have shown that 18 of the 20 amino acids can be synthesized from several different types of starting materials and energy sources. Though not specifically noted in the table, the molar yields vary dramatically when one compares the starting materials of the various experiments. Abelson found that when high proportions of methane and ammonia were mixed with water vapor and treated in an apparatus similar to Miller’s, no amino acids were produced.
In comparing the various problems of a prebiotic synthesis, an innate difficulty becomes apparent. Simply stated, the different conditions under which the various components of a living system are first thought to have arisen are in conflict with each other. Many diverse environments had to exist independently while allowing the products to be mutually dependent on one another, the resultants then coalescing and ultimately creating a living cell. This may be an understatement of the problem. One laboratory procedure for synthesizing prebiotic compounds will use one molar ratio of reactants while these will be varied for other procedures. Still others use dry concentrated reactants. Concentrating raw amino acids into com- pounds similar in purity to those used in the laboratory poses a further dilemma to the biochemical evolutionist. One environment, while capable of creating some of the amino acids and cellular building blocks, ultimately destroys many of the other components needed for the assemblage of a living cell. For instance, sugars are destroyed in an alkaline environment, the prominent environment in which the amino acids are produced. While some investigators assume basic conditions that are necessary for their synthesis of fundamental biological compounds, others employ acidic conditions. Also researchers have, at times, used different temperatures during the course of their reactions. Thus it becomes necessary to propose a highly complex and implausible model of the primitive earth.
To complicate matters further, one must account for the optical activity of all biological compounds; namely, the presence of L-α-amino acids and D-nucleosides. Present researchers believe this specificity to be purely accidental.7 A valid reason for the choice of one configuration over another has not yet been found. If the choice of the optical specificity of the amino acids and nucleosides is considered to be accidental, one must still be able to account for the incorporation into proteins of one kind of amino acid in preference to another.
Polymerization reactions present several problems. While they require unusually high concentrations of amino acids, the yields have been very low. It is difficult to conceive of a primitive-earth environment which would produce pure mixtures of amino acids rich in glutamic and aspartic acids as proposed. Secondly, polymerization only succeeds when mixtures are heated for short periods of time. Rapid cooling would be necessary. Other conditions do not produce proteinoids.
As for the synthesis of nucleic acids, phosphorylation and the subsequent formation of sugars, one major difficulty has not been previously stated. In the presence of ammonia (which is considered to have been a constituent of a reducing atmosphere), formaldehyde together with hydrogen cyanide forms glycine rather than condensing to produce a sugar. In effect, no sugars are formed.
In retrospect, those who investigate the origins of life in an evolutionary context should be asked to turn to new vistas, not in terms of men and machines, but to something beyond the understanding of life in chemical terms. Biochemical evolution is a feeble attempt at explaining the origin of life. From a scientific standpoint, this explanation leaves a large number of unanswered questions.
The fact that a chemist can carry out an organic synthesis in the laboratory does not prove that the same synthesis will occur in the atmosphere or open sea without the chemist. The second law of thermodynamics applies not only to inorganic gases in the atmosphere but also to organic compounds in the ocean. Living cells may reverse the process, but in the absence of life, ‘die Entropie der Welt stebt einem Maximum zu.’ [18]
As mentioned above, the experimental facts and accomplishments are at best minimal. Even if pure L-amino acids could have been synthesized, and even if they were polymerized into polypeptides of specific sequences, this still would be a long way from having all the proteins present in a single living cell. The same problem exists with the formation of nucleic acids, in which thousands of nucleotides must be joined in a very specific sequence; yet only dinucleotides have been formed under prebiotic conditions.
But even if proteins and nucleic acids could have been unequivocally synthesized by these experiments, their existence does not constitute a living system. A simple cell is an exceedingly complex ordered system. It has an amazing amount of information stored into its nucleus, information that determines the structure and function of the cell. It can reproduce itself, forming an identical twin, or it can differentiate. It has the ability to utilize and transform energy as well as store it for later use. All of these functions require a complex network of various integrated pathways involving a considerable number of chemical reactions, each one catalyzed by a specific enzyme. All the steps are carefully controlled by remarkable feedback mechanisms reminiscent of the operation of computers.
In the final analysis, the entire range of chemical evolution is one for which the following statement by Kerkut is particularly fitting:
It is very depressing to find that many subjects are becoming encased in scientific dogmatism. The basic information is frequently overlooked or ignored and opinions become repeated so often and so loudly that they take on the tone of laws. Although it does take a considerable amount of time, it is essential that the basic information is frequently re-examined and the conclusion analyzed. From time to time, one must stop and attempt to think things out for oneself instead of just accepting the most widely quoted viewpoint. [90]
This is what we have attempted to accomplish in this study. We have tried to carefully examine the scientific data presented in the literature dealing with chemical evolution and critically evaluate the results to determine if the conclusions of the investigators are sound. Such a study reveals that chemical evolution does not provide a satisfying solution to the question of the origin of life.
REFERENCES
[1]Charles Darwin C. 1890. The origin of species by means of natural selection. 6th reprint. NY: D. Appleton and Co. New York.
[2](a) Oparin AI. 1924. Proischogdenie Zhizni. Moscow: Moscovsky Rabotchii. (b) Oparin AI. 1938. The origin of life. NY: Macmillan Company.
[3](a) Haldane JBS. 1929. Rationalist Annual 148:3; (b) Haldane JBS. 1933. Science and human life. NY: Harper Brothers, p 148.
[4]Oparin AI. 1957. The origin of life on earth. NY: Academic Press.
[5]Oparin AI. 1953. The origin of life. 2nd English edition. NY: Dover Publications, p 101.
[6]Miller SL. 1953. A production of amino acids under possible primitive earth conditions. Science 117:528-529.
[7]Miller SL, OrgelLE. 1974. The origins of life on the earth. Englewood Cliffs, NJ: Prentice-Hall.
[8]Lemmon RM. 1970. Chemical evolution. Chemical Reviews 70:95.
[9]Abelson PH. 1966. Chemical events on the primitive earth. Proceedings of the National Academy of Sciences (USA) 55:1365-1372.
[10]Cloud PE, Jr. 1968. Atmospheric and hydrospheric evolution on the primitive earth. Science 160:729.
[11]Rutten MG. 1962. The geological aspects of the origin of life on earth. Amsterdam: Elsevier Publishing, p 106.
[12]Kenyon DH, Steinman G. 1969. Biochemical predestination. NY: McGraw-Hill.
[13]Miller SL, Urey HC. 1959. Organic compound synthesis on the primitive earth. Science 130:245-251.
[14]Urey HC. 1953. Proceedings of the Royal Society of London 219A:281.
[15]Ring D, et al. 1972. Prebiotic synthesis of hydrophobic and protein amino acids. Proceedings of the National Academy of Sciences (USA) 69:765-768.
[16]Gish DT. 1972. Speculations and experiments related to theories on the origin of life. San Diego, CA: Institute for Creation Research.
[17]Bernal JD. 1960. Thermodynamics and kinetics of spontaneous generation. Nature 186:694-695.
[18]Hull DE. 1960. Thermodynamics and kinetics of spontaneous generation. Nature 186:693-694.
[19]LawlessJ G, Boynton CD. 1973. Thermal synthesis of amino acids from a simulated primitive atmosphere. Nature 243:405-407.
[20]Fox SW, et al. 1963. Amino acid compositions of proteinoids. Archives of Biochemistry and Biophysics 102:439-445.
[21]Krampitz G. 1962. In: Stahmann MA, editor. Polyamino acids, polypeptides and proteins. Madison: University of Wisconsin Press.
[22]Fox SW, Harada KW. 1960. The thermal copolymerization of amino acids common to protein. American Chemical Society Journal 82:3745-3751.
[23]Fox SW. 1965. A theory of macromolecular and cellular origins. Nature 205:328- 340.
[24]Fox SW, editor. 1965. The origins of prebiological systems and of their molecular matrices. NY: Academic Press, p 361.
[25]Fox SW, HaradaK. 1960. Thermal copolymerization of amino acids in the presence of phosphoric acid. Archives of Biochemistry and Biophysics 86:281-285.
[26]Vegotsky A, Fox SW. 1959. Pyropolymerization of amino acids to proteinoids with phosphoric acid or polyphosphoric acid. Federation Proceedings 18:343.
[27]Steinman G, Lemmon RM, Calvin M. 1964. Cyanamide: a possible key compound in chemical evolution. Proceedings of the National Academy of Sciences (USA) 52:2730.
[28]Steinman G, Kenyon DH, Calvin M. 1965. Dehydration condensation in aqueous solution. Nature 206:707-708.
[29]Wald G. 1957. The origin of optical activity. New York Academy of Sciences Annals 69:352-368.
[30](a) Kuhn W, Braun E. 1929. Naturwissenschaften 17:227; (b) Michell S. 1930. Journal Chemical Society 20:1829; (c) Kuhn W, Knopf E. 1930. Naturwissen-schaften 18:183.
[31]SeifertH. 1956. In: Bechner B, editor. Von Unbelebten zum Lebendigen. Stuttgart: F. E. Verlagslrich Handling, p 68.
[32]Blout ER, Doty P, Yang JT. 1957. Peptides. XII. The optical rotation and configurational stability of a-helices. American Chemical Society Journal 79:749-750.
[33](a) Blout ER, Idelson M. 1956. Polypeptides. VI. Poly-a-L-glutamic acid: preparation and helix-coil conversions. American Chemical Society Journal 78:497-498;(b) Blout ER, Idelson M. 1956. Polypeptides. IX. The kinetics of strong-base initiated polymerizations of amino acid-N-carboxyanhydrides. American Chemical Society Journal 78:3857-3858.
[34]G. Steinman. 1967. Stereo selectivity in peptide synthesis under simple conditions. Experientia 23:177-178.
[35]Kovacs J, Kisfaludy L, Ceprini MW. 1967. On the optical purity of peptide active esters prepared by N,N’-dicyclohexylcarbodiimide and “complexes” of N,N’- dicyclohexylcarbodiimide-pentachlorophenol and N,N’-dicyclohexylcarbodiimide-pentafluorophenol. American Chemical Society Journal 89:183-184.
[36]Northrup JH. 1957. Optically active compounds from racemic mixtures by means of random distribution. Proceedings of the National Academy of Sciences (USA) 43:304-305.
[37]White A, Handler P, Smith EL. 1968. Principles of biochemistry. NY: McGraw- Hill.
[38]Oro J, Kimball AP. 1961. Synthesis of purines under possible primitive earth conditions. I. Adenine from hydrogen cyanide. Archives of Biochemistry and Biophysics 94:217-227.
[39]Ferris JP, Sanchez RA, Orgel LE. 1968. Studies in prebiotic synthesis. III. Synthesis of pyrimidines from cyanoacetylene and cyanate. Journal of Molecular Biology 33:693-704.
[40]Kemp I, Korhnstorm G. 1956. Journal Chemical Society 46:900.
[41]Sanchez RA, Ferris JP, Orgel LE. 1966. Cyanoacetylene in prebiotic synthesis. Science 154:784-785.
[42]Beck A, Lohrmann R, Orgel LE. 1967. Phosphorylation with in organic phosphates at moderate temperatures. Science 157:952.
[43]Miller SL. 1957. The formation of organic compounds on the primitive earth. New York Academy of Sciences Annals 69:260-274.
[44]Loeb W. 1913. Berichteder Deutsche Bunsengesellschaft für Physikalische Chemie 46:684.
[45]Baly E, Heilbron I, Hudson D. 1922. Photocatalyses, the photosynthesis of nitrogen compounds from nitrates and carbon dioxide. Journal Chemical Society 12:1078. 46.
[46]Garrison WM, et al. 1951. Reduction of carbon dioxide in aqueous solutions by ionizing radiation. Science 114:416-418.
[47]Bahadur K. 1954. Photosynthesis of amino-acids from paraformaldehyde and potassium nitrate. Nature 173:1141.
[48]Hough L, Rogers AF. 1956. Synthesis of amino-acids from water, hydrogen, methane and ammonia. Journal of Physiology 132:28P-30P.
[49]Paschke R, R. Chang RWH, Young D. 1957. Probable role of gamma irradiation in origin of life. Science 125:881.
[50]Abelson PH. 1957. Discussionof S.L. Miller’s The formation of organic compounds on the primitive earth. New York Academy of Sciences Annals 69:274-275.
[51]Santamaria L, Fleischmann L. 1966. Photochemical synthesis of amino acids from paraformaldehyde catalyzed by inorganic agents. Experientia 22:430-431.
[52]Bahadur K, Ranganayaki S, Santamaria L. 1958. Photosynthesis of amino-acids from paraformaldehyde involving the fixation of nitrogen in the presence of colloidal molybdenum oxide as catalyst. Nature 182:1668.
[53]Deschreider AR. 1958. Photosynthesis of amino-acids. Nature 182:528.
[54]Reid C. 1959. Proceedings of the international symposium on the origin of life on earth. Vol. 1, p 619-625. NY: Pergamon Press.
[55]Pavlovskaya TE, Pasynskii AG. 1959. Proceedings of the international symposium of the origin of life on earth. Vol. 1, p 151-157. NY: Pergamon Press.
[56]Oro J, et al. 1959. Amino acid synthesis from formaldehyde and hydroxylamine. Archives of Biochemistry and Biophysics 85:115-130.
[57]Fox SW. 1960. How did life begin? Science132:200-208.
[58]Lu HK, et al. 1960. Formation of sulfur-containing amino acids by electric discharge in a reductive atmosphere. Chemical Abstracts 54:4209-4210.
[59]Oro J, Kamat SS. 1961. Amino-acid synthesis from hydrogencyanide under possible primitive earth conditions. Nature 190:442-443.
[60]Fox SW. 1961. The origins of pre-biological systems and of their molecular matrices. NY: Academic Press, p 137-162.
[61]Palm C, Calvin M. 1962. Primordial organic chemistry. I. Compounds resulting from electron irradiation of C14H4. American Chemical Society Journal 84:2115-2121.
[62]Lowe C, Rees M, Markham R. 1963. Synthesis of complex organic compounds from simple precursors: formation of amino-acids, amino-acid polymers, fatty acids and purines from ammonium cyanide. Nature 190:219.
[63]Oro J. 1963. Synthesis of organic compounds by electric discharges. Nature 197:862-867.
[64]Steinman GD, Lillevik HA. 1964. A biotic synthesis of amino groups. Archives of Biochemistry and Biophysics 105:303-307.
[65]Harada K, Fox SW. 1964. Thermal synthesis of natural amino-acids from a postulated primitive terrestrial atmosphere. Nature 201:335-336.
[66]Kolomiychenko MA. 1965. Photochemical synthesis of amino acids. Federation Proceedings (Translation Supplement) 24:T199-T202.
[67]Matthews CN, Moser RE. 1966. Prebiological protein synthesis. Proceedings of the National Academy of Sciences (USA) 56:1087-1094.
[68]Choughuley ASU, Lemmon RM. 1966. Production of cysteic acid, taurine and cystamine under primitive earth conditions. Nature 210:628-629.
[69]Matthews CN, Moser RE. 1967. Peptide synthesis from hydrogen cyanide and water. Nature 215:1230-1234.
[70]Harada K. 1967. Formation of amino-acids by thermal decomposition of formamide — oligomerization of hydrogen cyanide. Nature 214:479-480.
[71]Hasselstrom T, Henry MC, Munn B. 1957. Synthesis of amino acids by beta radiation. Science 125:350-351.
[72]Dose K, Ponnamperuma C. 1967. The effect of ionizing radiation on N-acetylglycine in the presence of ammonia. Radiation Research 31:650-651.
[73]Moser RE, Claggett A, Matthews CN. 1968. Peptide formation from diaminomaleo- nitrile (HCN tetramer). Tetrahedron Letters 13:1599.
[74]Moser RE, Matthews CN. 1968. Hydrolysis of aminoacetonitrile: peptide formation. Experientia 24:658-659.
[75]Steinman G, Smith AE, Silver JJ. 1968. Synthesis of asulfur-containing amino acid under simulated prebiotic conditions. Science 159:1108-1109.
[76]Friedmann N, Miller SL. 1969. Phenylalanine and tyrosine synthesis under primitive earth conditions. Science 166:766-767.
[77]Friedmann N, Miller SL. 1969. Synthesis of valine and isoleucine in primitive earth conditions. Nature 221:1152-1153.
[78]Bar-Nun A, et al. 1970. Shock synthesis of amino acids in simulated primitive environments. Science 168:470-473.
[79]Fox SW, et al. 1970. Bio-organic compounds and glassy microparticles in lunar fines and other materials. Science 167:767-770.
[80]Nagy B, et al. 1970. Organic compounds in lunar samples: pyrolysis products, hydrocarbons, amino acids. Science 167:770-773.
[81]Fox SW, Windsor CR. 1970. Synthesis of amino acids by the heating of formaldehyde and ammonia. Science 170:984-986.
[82]Khare BN, Sagen C. 1971. Synthesis of cystine in simulated primitive conditions. Nature 232:577-579.
[83]Sagen C, Khare BN. 1971. Long-wave length ultraviolet photo production of amino acids on the primitive earth. Science 173:417-420.
[84]Trump JEV, Miller SL. 1972. Prebiotic synthesis of methionine.Science178:859- 860.
[85]Ferris JP, Donner DB, Lobo AP. 1973. Possible role of hydrogen cyanide in chemical evolution: investigation of the proposed direct synthesis of peptides from hydrogen cyanide. Journal of Molecular Biology 74:499-510.
[86]Ferris JP, Donner DB, Lobo AP. 1973. Possible role of hydrogen cyanide in chemical evolution: the oligomerization and condensation of hydrogen cyanide. Journal of Molecular Biology 74:511-518.
[87]Harada K, Iwasaki T. 1974. Syntheses of amino acids from aliphatic carboxylic acid by glow discharge electrolysis. Nature 250:426-428.
[88]Ferris JP, et al. 1974. Chemical evolution; the amino acids released on hydrolysis of HCN oligomers. Journal of Molecular Evolution 3:225-231.
[89]Garay AS. 1968. Origin and role of optical isomery in life. Nature 219:338-340.
[90]G. A. Kerkut. 1960. Implications of evolution. Pergamon Press, New York, p, viii.