©Copyright 2018 GEOSCIENCE RESEARCH INSTITUTE
11060 Campus Street • Loma Linda, California 92350 • 909-558-4548

CHAPTER THREE: WHAT MAKES A CELL TICK?
by
George T. Javor
Loma Linda University
“Old chemists never die, they just reach equilibrium”.
In presenting a case for a tight logical link between analyzing the molecular aspects of life and the creationist paradigm, it is not enough to enumerate the components of living matter. Simply knowing the components of living matter is not enough to account for its biological activity. Living matter behaves differently than its isolated components. Living cells incorporate selected substances and utilize them either for energy or as building blocks for growth. They also secrete metabolic waste. Living cells grow and divide into daughter cells. Lastly, when cells recognize unfavorable environmental conditions, they make metabolic adjustments to preserve their existence. [1] Living matter gives every indication that it “wants” to stay alive. This is a property of the complex network of components in living matter. The whole seems to be more than the sum of its parts. If we collect all of the ingredients from live cells, lace them in a membrane-enclosed vesicle, we have an inert, “lifeless” assembly of biomatter. This bag may be stored indefinitely in an environment hospitable for life, without the actual emergence of life. If we periodically analyzed the contents of this artificial “cell”, we would find little change in its chemical composition. Such an arrangement of matter is called equilibrium. [2]
If we sampled the composition of life cells growing in a defined laboratory setting, surprisingly, the results would be similar, That is, we would find the chemical composition of live cells quite constant. But instead of the term “equilibrium”, we say that matter in live cells is in a “steady state system”. The significant difference between the two is the dynamic flux of matter through live cells.
A mechanical illustration of this difference is shown in Figure 3.1.
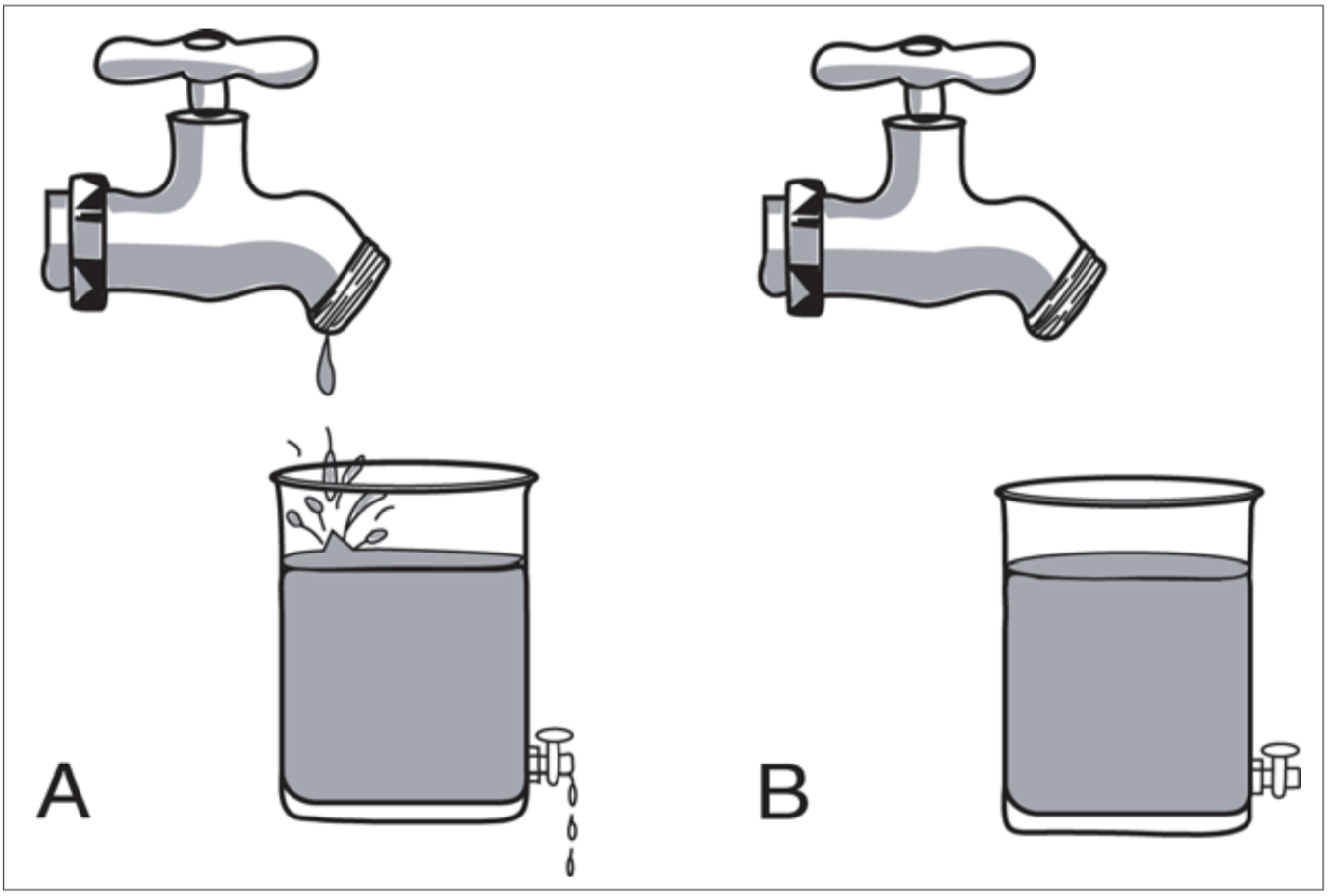
Here, the contents of both vessels remain unchanged over time, but there is constant movement of liquid through vessel A. The flow of molecules through cells is an essential feature of life. (In contrast, the liquid in container B is stagnant.) The movement of water through a compartment, representing the flux of matter through the cell, is an oversimplification of what actually occurs. In reality matter changes as it travels through the cell. The incoming precursors (biomonomers)are simple substances which are gradually built up to successively more complex structures. In Figure 3.2 this is represented by the arrows on the right, marked “biosynthesis” and “assembly”.
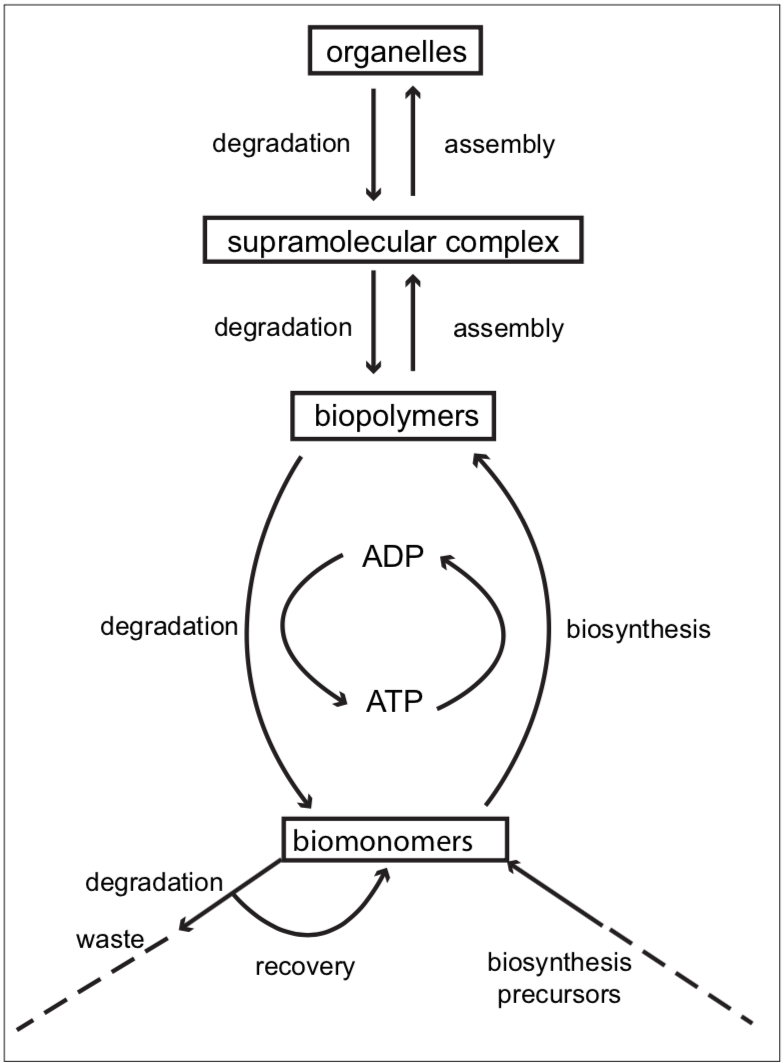
FIGURE 3.2. Movement of matter through the cell.
To complete the circuit, the biosynthetic flow of matter is balanced by a set of degradative pathways. The very existence of degradative pathways in the cell is remarkable, in view of the fact that biopolymers and the successively more complex structures are made at prodigious expenditure of energy. Their constant degradation and remodeling would seem a phenomenal waste. But we now know that in the course of metabolism, components sustain oxidative damage with time. Accumulation of damaged metabolites would clog the cell’s machinery. The constant turnover of biomatter preempts such a scenario.
However, since both biosynthesis and degradation are occurring in the same cell, the two processes need to balance. An excess rate of degradation over biosynthesis would be particularly disastrous. Thus, the rates of all metabolic processes have to be coordinated for this tightrope act of metabolic symmetry (steady state). Figure 3.2 also shows the linkage between energy usage and biosynthesis. The substance abbreviated as “ATP” is the chief carrier of chemical energy in the cell. Most frequently, when chemical change requires input of energy, ATP (adenosine triphosphate) is degraded to ADP (adenosine diphosphate). The sum total of the chemical changes in the cell equals the essence of life.
WHAT ARE CHEMICAL REACTIONS, ANYWAY?
Chemical reactions are nothing more than the movement of bonding electrons around and between atoms. These electrons hold groups of atoms in clusters called molecules. The fascinating property of matter is that these clusters behave very differently than their constituent atoms. [4] For example, clusters of oxygen atoms and clusters of hydrogen atoms by themselves are gases. Hydrogen is very flammable, even explosive. But when an oxygen and two hydrogen atoms are combined into a cluster, water forms. The conversion of a mixture of oxygen gas and hydrogen gas to water is a chemical reaction.
In chemical reactions, atoms and their bonding electrons leave an old cluster and join a new one. As a result of changes in their atomic compositions, the chemical properties of clusters change. By “chemical property” we mean the tendency of molecules to acquire or give up atoms.
Why would atoms and their bonding electrons jump from one atomic cluster to another? This is equivalent to asking why chemical reactions occur at all. Chemists tell us that the driving force behind chemical changes is the intrinsic tendency of all matter to exist in the lowest possible state of energy. This is why balls roll downhill spontaneously, electricity flows from the negative to the positive pole, and hot objects tend to cool down to the temperature of their environment.
Chemical reactions can be compared to a market transaction where molecules trade atoms in order to shed some of their energy content. Just as in business there are sellers and buyers, in chemical reactions there are atom clusters that can achieve lower energy states by rearranging or giving up some of their atoms. These are the “sellers”. Other clusters — the “buyers” — receive new atoms, and their energy levels increase. The important consideration for such chemical transactions to occur is that when all the energy gains and losses are totaled, there should be a net lowering of the overall energy content of the system.
Chemical changes take a finite amount of time. Some rearrangements are faster than others. Chemists have found that if the vibrations of atoms are speeded up by raising the temperature, the chemical change is more rapid. There are also helper agents — catalysts — which facilitate reactions. Remarkably, almost every chemical change in the cell has a facilitator catalyst — an “enzyme”. Enzymes are very large protein molecules, often hundreds of times larger than the atomic groups they manage.
WHY ENZYMES?
The role of catalysts is the speeding up of chemical conversions. In the case of living matter, why are catalysts necessary? Why must there be an increase in reaction rate? If all chemical changes in the cell would slow down due to lack of catalysts, what would happen? The answer is: chaos. Without specific catalysts guiding molecules through precise paths of chemical changes, numerous “unauthorized” chemical side-reactions would occur. This is due to the propensity of substances to interact with each other in more than one way. Only those chemical changes which contribute to the economy of the whole cell are useful.
This is a significant point. Individual chemical changes in the cell (even dozens or hundreds of them) have little utility, unless their end products belong in the closely knit network of substances needed by the cell. The meaning of each chemical reaction and of each reaction product is derived from the fact that the reaction products contribute to the functions of the living cell. All other chemical conversions are wasteful and detrimental to the cell.
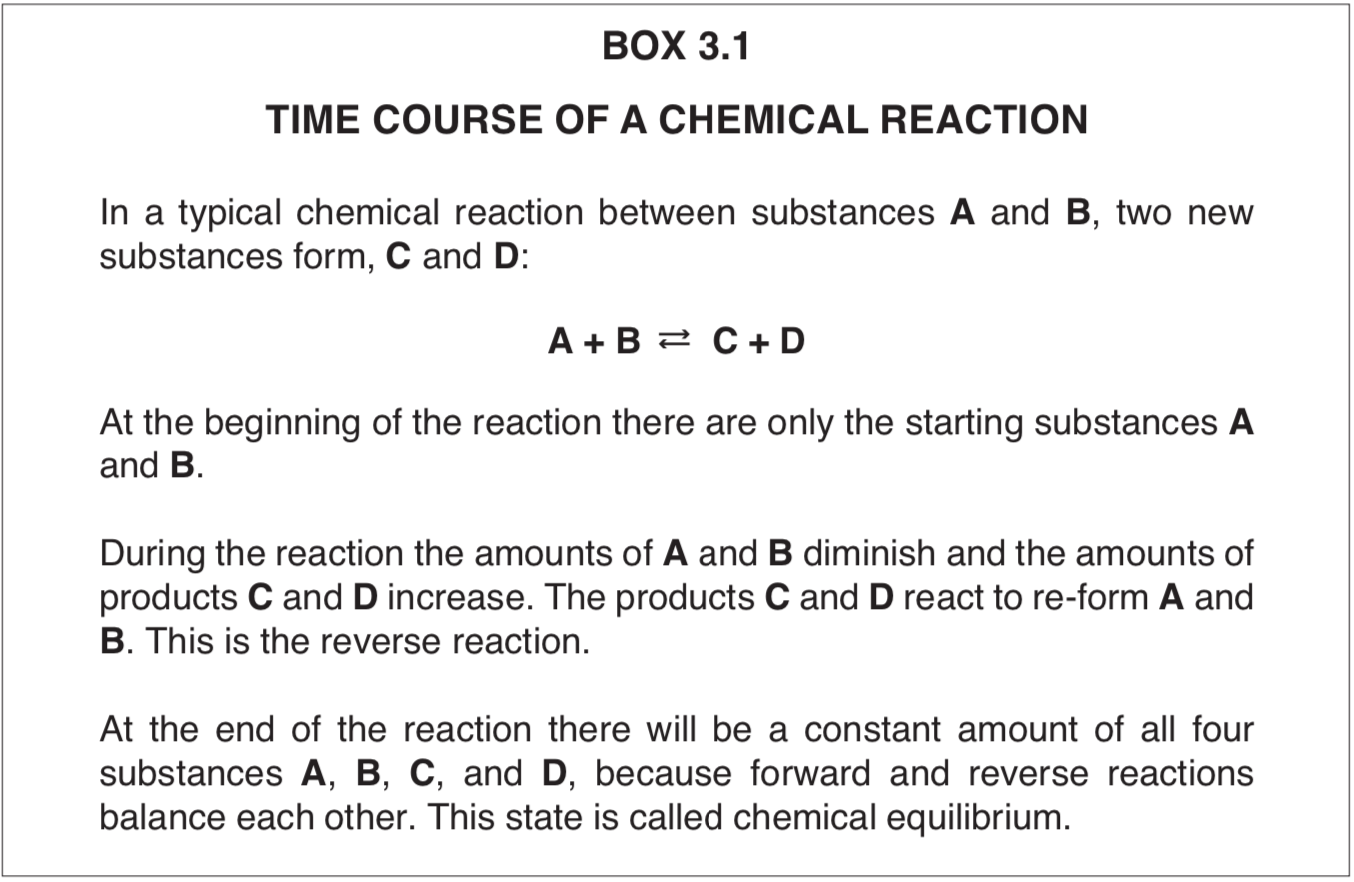
A reaction always runs its course, whether catalyzed or not, by producing a characteristic ratio of reaction products and starting materials. When this ratio is achieved, no further net chemical change happens under the conditions of the reaction, and the substances are said to be in a state of chemical equilibrium (Box 3.1).
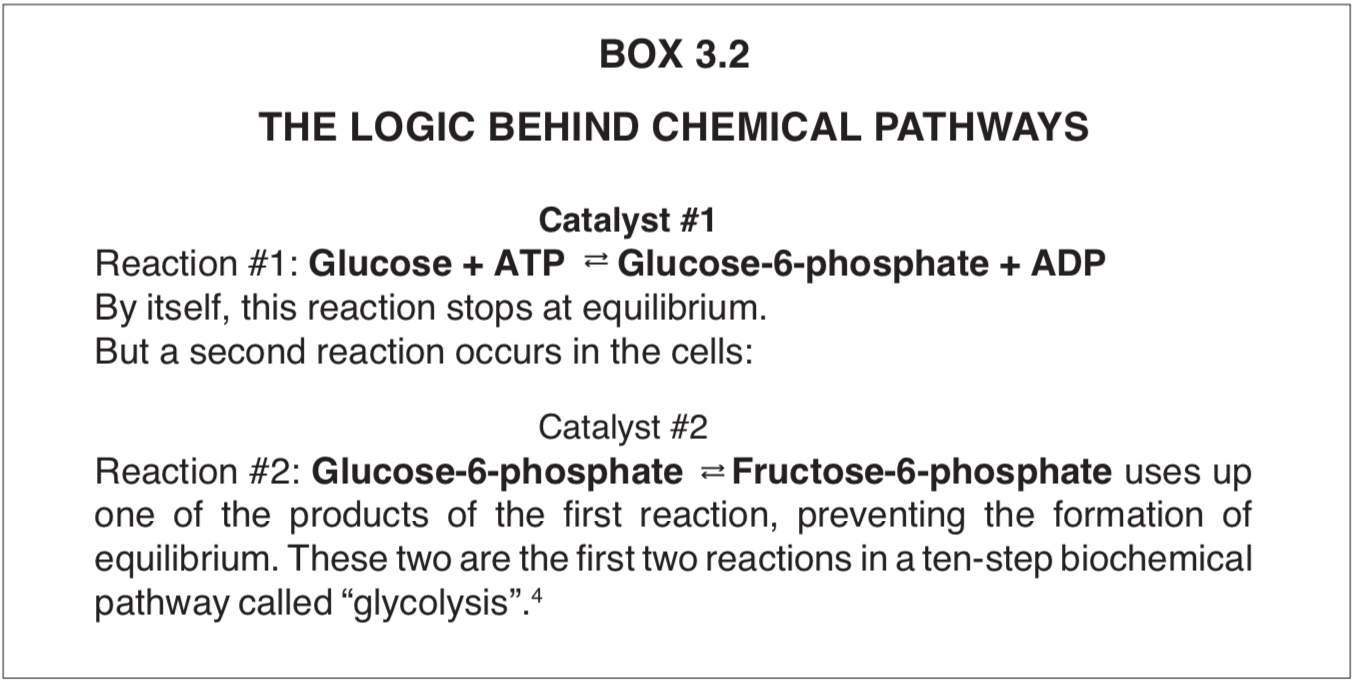
For every reaction there is an “equilibrium constant” Keq, a term which combines the characteristic ratios of reaction products and reactants at equilibrium. Reactions at equilibrium are of little use to the cell because it is the chemical changes that drive the phenomenon of life. In fact, when all of the reactions in the cell reach their equilibria, death occurs. This makes the roles of the enzymes paradoxical. They are required to keep the flow of materials on useful tracks preventing side-reactions, but the enzymes push the chemical conversions rapidly toward equilibria which, if achieved, doom the cell. To avoid disaster, the chemical conversions are organized into what amounts to “assembly lines” on the cell. The product of one reaction becomes the starting material for the next. This arrangement prevents the accumulation of products (Box 3.2).
In the hundreds of chemical assembly lines, also called “biochemical pathways”, there are multiple chemical conversions. Some of these build larger and larger molecules, while other pathways degrade substances to smaller pieces. Degradation of energy-rich matter is coupled to the efficient capture of chemical energy. This energy drives the growth and movement of the cell. Figure 3.2 is an attempt to summarize the work of the metabolic paths in the interacting networks.
The metabolic fabric of the cells is seamless; there are no loose ends. All biosynthetic paths lead to the production of more biomatter and growth, and all degradative processes result in the harness of useful chemical energy and in the secretion of waste. Each biochemical pathway has a single “rate limiting” step which governs the rate for output of that chemical “assembly line.” The enzyme catalyzing this regulatory reaction is able to speed up, slow down, or even arrest the output of that pathway, depending on the amount of product already available to the cell. Thus, wasteful oversupply of metabolic components is prevented. This is one of the kinds of sensing mechanisms which monitor the composition of the intracellular environment. As excesses or shortages of biochemical intermediates develop, appropriate regulatory adjustments are made in order to preserve the “steady state” of the cell. In a well-functioning cell, the amounts of each of hundreds or thousands of substances remain close to constant during a steady flux of material through the system. This steady, non-equilibrium state of matter is an absolute prerequisite for the phenomenon of life.
CHEMICAL DIFFERENCE BETWEEN LIFE AND DEATH
If a single reaction within a metabolic pathway were to reach equilibrium in the cell, it would constitute a metabolic block, because (by definition of what equilibrium is) there would be no net conversion of matter past that point. Some metabolic blocks would not be fatal when alternative pathways could compensate the cell. But when all the reactions in the cell reach their equilibria, life processes cease, and the cell dies. Such a state can be achieved in a bacterial cell such as E. coli, by using an organic solvent to poke holes in its membrane. When the membrane is opened the cell is no longer capable of generating energy (an intact membrane is essential for this process). The chemical conversions cease, and soon every reaction will reach its equilibrium. The difference between non-equilibrium and equilibrium is nothing less than the difference between life and death. [5]
The equilibrium status of a single chemical reaction can be converted into a non-equilibrium state temporarily by either adding more reactants or by removing one or more reaction products. The non-equilibrium state will last only as long as one of these measures continues. The same considerations apply to biochemical chain reactions (pathways) that have reached equilibrium. The equilibrium of each pathway may be eliminated if it is provided with additional starting material and if the final product is removed. This concept is illustrated in Box 3.3.
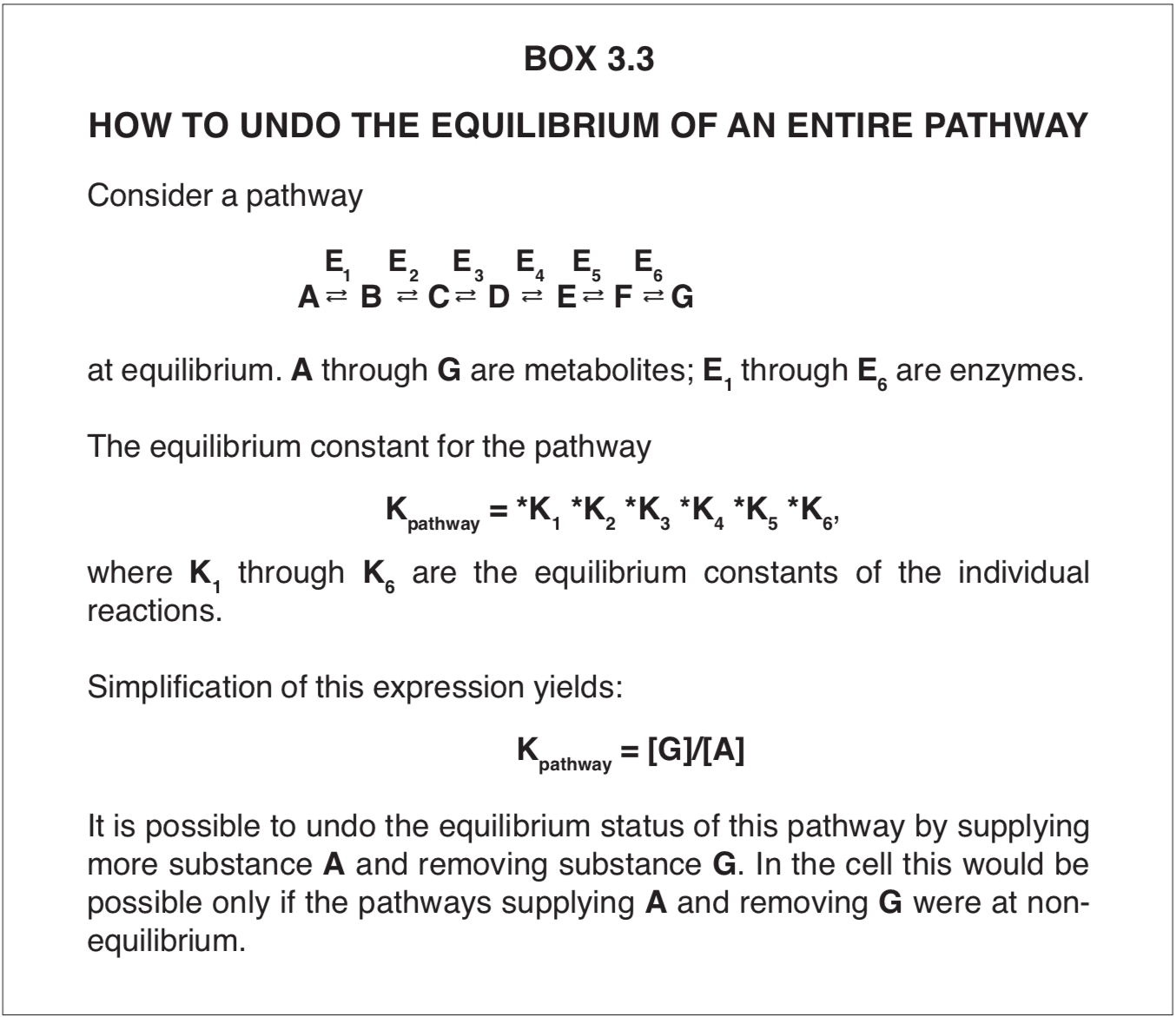
Thus, if the non-equilibrium steady states of all reactions could be restored, the dead cells would live again. In theory this could be accomplished by restoring each of the hundreds of interconnected biochemical pathways to its non-equilibrium condition (Box 3.3). First any breach of the integrity of the cell’s membranes would have to be repaired, and a continuous supply of starting substrates of the first reactions of every pathway would have to be supplied in order to launch all of the chemical processes, more or less simultaneously. Such a procedure would begin turning the metabolic wheels of the organisms, and it would live again.
While we can transfer any substance across a cell membrane by “electroporation” (a short pulse of high voltage), [6] the continuous delivery into cells of large numbers of different metabolites for which there are no built-in transport mechanisms is beyond our current technical capabilities. Herein lies the reason why we cannot reverse death on the cellular level.
Closely akin to this is the problem of generating life from an inert collection of biomolecules. To accomplish this, one would need to bring all of the needed substances into a membrane-enclosed space (enzymes, substrates, genetic material, various subcellular organelles) and then create a state of non-equilibrium among the hundreds of substrates of the enzymes. The difficulty in accomplishing this rests with the propensity of enzymes to establish equilibrium rapidly among their substrates. Thus far it has not been possible to overcome this challenge even in the most sophisticated modern laboratory. What would be required here is to be able to manipulate selected molecules in the manner of “Maxwell’s Demon.”
HOW MAXWELL’S DEMON WORKS
This theoretical creature occupied space between two interconnected compartments which were filled with gas molecules, and the demon could keep the slow-moving molecules in one compartment and send the fast-moving ones into the other. Such action would result in one compartment becoming warm and the other cold. In other words, Maxwell’s Demon could take a system at equilibrium and manipulate it into a non-equilibrium state.
Whether this feat can be accomplished in the future by scientists is not known. Manipulation of individual atoms and molecules is now becoming possible, using “atomic force” microscopy. [7] It is certain, however, that a state of non-equilibrium cannot arise from an equilibrium state spontaneously. But this is precisely what would be required if a live cell were to emerge from a dead cell (Box 3.4).
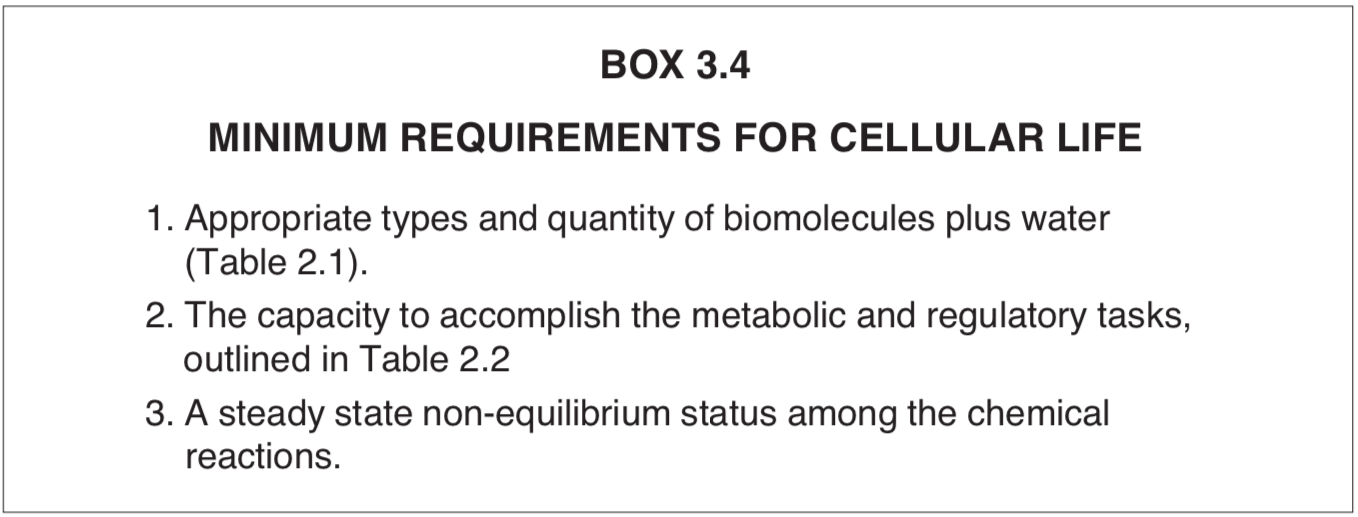
The discussion of life in these three chapters emphasized both the complexity and the dynamics of the chemistry undergirding this amazing process. The minimum requirements for cellular life are summarized in Box 3.4. With these facts in the background we now turn to the current postulates of primordial abiogenesis that attempt to explain how life arose on Earth from nonliving matter.
SUMMARY OF CHAPTER 3
- Living cells are made from nonliving components.
- The phenomenon of life is based on continuous chemical conversions.
- Individual chemical reactions always reach their end points (equilibrium) and come to a stop.
- In living cells, chemical reactions are linked into chains that prevent individual reactions from reaching equilibrium and stopping.
- The difference between live and dead cells is the equilibrium or non-equilibrium status of the chain reactions.
- At the present time, even with our considerable chemical knowledge, we cannot restore dead cells back to life.
REFERENCES AND NOTES ON CHAPTER 3
[1]Lehninger AL, Nelson DL, Cox MM. 1993. Principles of biochemistry. Second ed. NY: Worth Publishers, p 3.
[2]Equilibrium does not have to be a state of stagnation. There can be many chemical reactions occurring in such a system, the only requirement is that there will be no net chemical change. In other words, in a state of equilibrium, the various chemical changes cancel each other.
[3]The original Figure 3.1 was drawn by Mrs. Anita Churches.
[4]Timberlake KC. 1999. Chemistry: an introduction to general, organic and biological chemistry. Seventh ed. Menlo Park, CA and NY: Addison-Wesley Longman, Inc.
[5]Becker WM. 1977. Energy and the living cell. Philadelphia and NY: J.B. Lippincott Co., p 32.
[6]Maniatis T, Sambrook J, Fritsch EF. 1989. Molecular cloning: a laboratory manual. Second ed. 3 vols. NY: Cold Spring Harbor Laboratory Press.
[7]Mathews CK, Van Holde KE. 1996. Biochemistry. Second ed. Menlo Park, CA and NY: The Benjamin/Cummings Publishing Co., p 23.