©Copyright 2018 GEOSCIENCE RESEARCH INSTITUTE
11060 Campus Street • Loma Linda, California 92350 • 909-558-4548

CHAPTER TWO: THE MATTER OF LIFE AND DEATH
by
George T. Javor
Loma Linda University
“Material things I touch and taste and see, all other things are immaterial to me”.
A. Bierce
What is the difference between living and inanimate matter? Intuitively, we are confident that we can easily tell the difference between them. But, with the rapid increase in computer technology, could it happen that artificial intelligence one day will effectively mimic aspects of human reasoning and behavior? Such scenarios have already been presented in science fiction, where humans had to deal with a supercomputer “Hal” in 2001, a Space Odyssey, or with lifelike robots in the tale The Stepford Wives. What criteria would we use in deciding that those moving and talking mannequins were lifeless after all?
Paul Weiss, a well-known biologist, wrote a tongue-in-cheek piece entitled “Life on Earth (by a Martian)”.1 In this story, some Martians came to visit Earth in search of life. After lengthy and careful observation of our planet, they concluded that life did indeed exist here, and furthermore, one life form was predominant. They named it the “Earthian” and faithfully chronicled its every particular. Apparently the Earthians had intricate symmetrical bodies; they moved, emitted heat and sounds, and ate (mostly liquid food). Sometimes they divided, and they eventually died. Which organism did the Martians observe and describe? Automobiles, of course! (The Martians also noticed some rather unimpressive structures associated with the Earthians, and they concluded that these were some sort of parasites, unworthy of further study.)
Our capacity to determine whether or not an entity is alive is limited by our previous experiences with living organisms. If we landed on a new planet, we could find it difficult to decide whether or not life was present.
LOOKING FOR LIFE ON MARS
In the 1970s the United States sent two automated laboratories to Mars to determine if living organisms existed there. The results of the biology experiments strongly suggested some kind of biological activity on Mars. Carbon dioxide was released when a nutrient-rich liquid mixture was incubated with a scoop of Martian soil. In an Earth-based laboratory, the results would have constituted compelling evidence for the presence of life. Yet scientists interpreting the data which was radioed back from Mars were forced to conclude that in all likelihood Mars was sterile. The reason was that chemical analyses of the Martian surface indicated the complete lack of carbon-containing substances, other than the gas carbon dioxide.2 Now we know that the iron-rich surface, activated by ultraviolet radiation, degraded the radioactive test substances, resulting in false chemical signals. This is an example of carefully designed experiments, aimed at distinguishing between the presence or absence of life on the red planet, that were not quite equal to the task.
IS THERE A DIFFERENCE BETWEEN LIVING AND NONLIVING MATTER?
There are those who see an unbroken continuum between living and nonliving matter. If this is so, the question of life’s origin becomes a moot point. Viruses, prions, mycoplasmas, rickettsiae and chlamidiae are offered as examples of organisms that bridge the chasm between living and nonliving. But the differences between living and nonliving matter are in fact so great that this chasm cannot be spanned.
Although viruses and prions are made from biopolymers, they are no more alive than the enzyme additives in some detergents. Viruses are lifeless complexes of proteins and nucleic acids. The biological activity of viruses, including their replication, is completely dependent on the metabolic activity of the infected cell. Prions are unique proteins that alter the structure of certain other proteins. The newly changed proteins in turn acquire prion-type activity, creating a domino effect of protein alteration. This property of prions renders them infectious. For reproduction, prions, like viruses, are wholly dependent on live cells.
Rickettsiae, chlamidiae and mycoplasmas, on the other hand, are among the smallest known living organisms, and are very much alive. The fact that chlamidiae and rickettsiae are obligate intracellular parasites only means that they have serious metabolic deficiencies. A clear distinction between living entities and nonliving substances is essential for a consideration of whether it is possible to go from one state to the other. For this reason we need to descend into the submicroscopic world of matter.
The elemental compositions of living and nonliving matter differ greatly.4 The actual chemical determination of living matter is done on “once-living matter”. Before chemists can analyze living matter, they have to take it apart to isolate its individual components, thereby killing it. Thus the actual phenomenon of “life” is not amenable to detailed chemical scrutiny. In the very process of laying hold of isolated “purified” components of living matter, “life” slips out between the chemists’ fingers, and what remains is an inert, “lifeless” substance. This is so because living cells are composed of lifeless, nonliving components. The implication is that the difference between life and death is a question of how biomatter is organized. Therefore, it should be possible to reverse the killing of cells by restoring them to their pre-disruption state. Why this has not yet been done in the laboratory will be discussed in the next chapter.
The chemical evolutionary issue can be reduced to answering a two-part question: 1) Is it conceivable that appropriate types of biomatter could have emerged on a hypothetical primordial earth; and 2) If these substances existed, could they have combined to form living matter?
Chemists have obtained valuable information regarding the differences in the composition of “once-living” matter and of “never-living” substances. Never-living matter — rocks, minerals, air, water, etc. — consists of small molecules, often with high oxygen content. These “oxides” are sturdy substances, stable under heat and mechanical stress. A good example is silicon dioxide — sand, a most abundant gritty stuff.
Living and once-living organic matter, in contrast, is predominantly constituted from large molecules which contain thousands, or even millions, of atoms. The oxygen content in these substances is low, but if oxygen is allowed to interact freely with these molecules, they lose biological activity. Surrounded by a sea of oxygen, living matter continually fights the inroads of this element with oxygen-neutralizing mechanisms. Fragile biomolecules are easily degraded or deformed by heat or mechanical stress.
The large qualitative differences between living and inanimate matter have been recognized for hundreds of years. Scientists initially thought that biological material could be produced only by living organisms, so they called these “organic”. But in 1828 the German chemist Frederich Wohler accidently produced urea by heating potassium cyanate with ammonium sulfate. Urea was at that time already recognized as an animal waste product, definitely “organic” in nature. Scientists quickly realized the implications of this breakthrough discovery. The production of biological matter did not, after all, depend on “life forces”. The term “organic” was retained to designate all compounds that contain carbon, with a few exceptions such as carbon monoxide, carbon dioxide, carbides, carbonates, cyanides and isocyanates.
Although the types of life-forms run into the millions, their general chemical compositions have important similarities. The gross chemical composition of the well-studied colonorganism Escherichiacoli represents the “typical cell” (see Table 2.1).
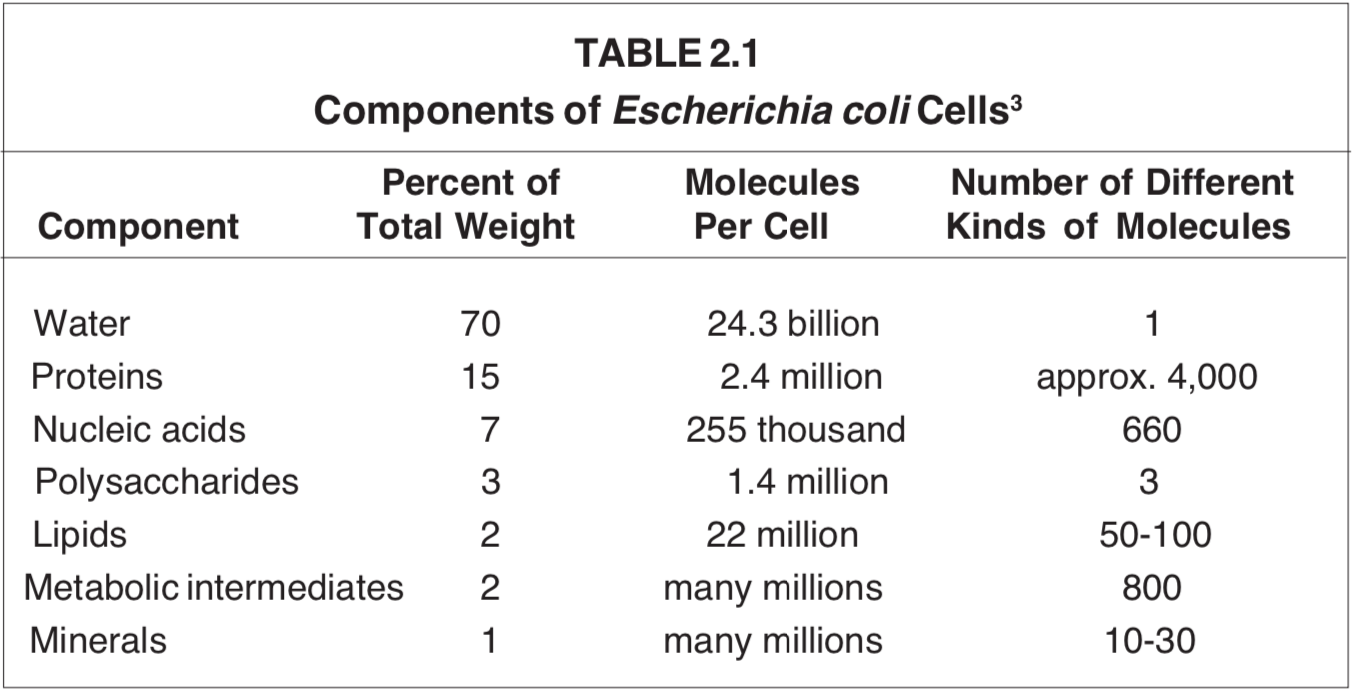
The high water content of living matter prompted Dr. Szent-Gyorgyi to write: “we are a walking aquarium”.5 We need all that water to enable most chemical transformations of life to take place.
The importance of water for life-processes can be demonstrated quite dramatically with freeze-dried microorganisms. We can collect bacteria from growth in a liquid medium in the form of a wet paste. Rapidly freezing this material and placing it under vacuum causes the frozen water to leave the bacteria unobtrusively, and the former wet paste turns into powder. The dried microscopic cells are now in a state of suspended animation. They are neither alive, nor are they dead. They can be stored indefinitely in the dried state, without any change in their status. However, by simply mixing the powder with water and the appropriate nutrients, the dormant cells spring into life once again. In this procedure we manipulate life on the cellular level by withdrawing an all-important cellular component. The most remarkable aspect of this process is the reversibility of the life-processes by manipulating only the water content of the cells!
HOW BIOPOLYMERS ARE PUT TOGETHER
The bulk of dry matter in all organisms (more than 90%) is composed of the biopolymers: proteins, nucleic acids, polysaccharides and lipids. A common feature of these four classes of substances is that they contain many repeats of small building-block substances. Very significantly, all chemical linkages between the building blocks are created by dehydration. That is, the building blocks of all biopolymers are linked by splitting out water between them. This information is summarized in Table 2.2.
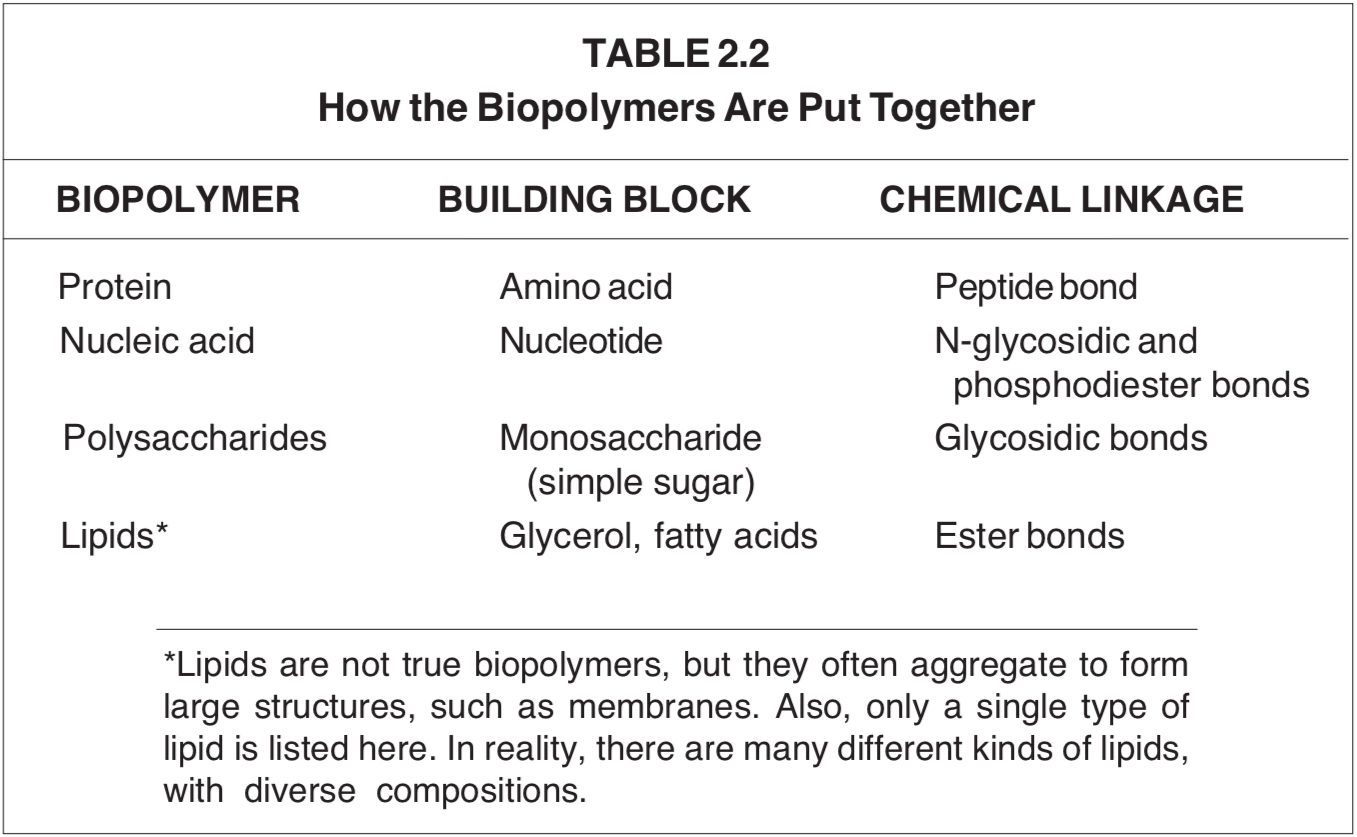
One of the challenges of chemical evolutionary postulates is to explain how these biopolymers could arise in a world assumed to be covered with water. It is a most difficult task to form new chemical bonds by eliminating water in an aqueous environment!
HOW CAN WE HAVE SO MANY DIFFERENT KINDS OF PROTEINS?
The bulk of biomatter is made from proteins. These are a most interesting and versatile class of materials. In each cell there are hundreds to thousands of different types of proteins, each with different chemical and physical characteristics. Such diversity is due to both their great size (they are composed typically of long strings of amino acids) and to the fact that any amino acid may be one of twenty different kinds.7 What each protein is capable of doing depends a great deal on the actual order in which the amino acids are linked. This feature of biology is similar to a written language.
A word’s meaning depends on the sequence of its letters. We choose from 26 letters of the English alphabet to make words. An estimated 500,000 different combinations of letters in our language are recognized as meaningful. With some effort, we could come up with many more sets of 500,000 combinations of letters that would be nonsensical (Dr. Seuss started a nice collection of these). Similarly, the millions of proteins represent only a tiny fraction of all possible combinations of amino acids.
Misspelling words jeopardizes their meaning. Likewise, for proteins to function properly their amino acids must follow each other in a correct order.8 For example, the oxygen-carrying component in our blood — hemoglobin — is built from 4 separate protein chains, each of which is a string of 142-146 amino acids. In an inherited illness called “sicklecell anemia”, the gene for one of the protein chains of hemoglobin sends out incorrect information to the protein-making complex. This results in placing a wrong amino acid in the sixth position of a specific sequence of the 146. This alteration is enough to lead to distortion of the red blood cell, to anemia, to many other problems, and, sadly, to death in many cases. While not all changes in amino-acid sequences have such drastic consequences, this somewhat extreme example underscores the importance of the correct order of amino acids for proteins.
The amino-acid sequences of proteins are crucial components of the biological information content of cells. Proteins themselves are considered informational biomolecules. But how does the proteinbuilding apparatus know the correct amino-acid sequence for each of the thousands of different proteins found in the cell?
The answer is that the genes of each cell are libraries containing just such information. When the cell needs to make a certain type of protein, it sends a copy of its amino-acid sequence information to the protein-synthesizing complex. Bacteria, with a thousand different proteins, have a minimum of a thousand genes. The recent triumph of obtaining the complete nucleotide sequence of organisms has enabled us to count their genes and even to assign a function to many of them. Table 2.3 shows a compilation for three microorganisms; Haemophilus influenzae (1743 genes), Mycoplasma genitalium (471 genes) and Escherichia coli (4288 genes). The first two organisms grow only inside humans in a nutritionally rich environment. Escherichia coli, on the other hand, can proliferate independently and may be considered a free-living organism.
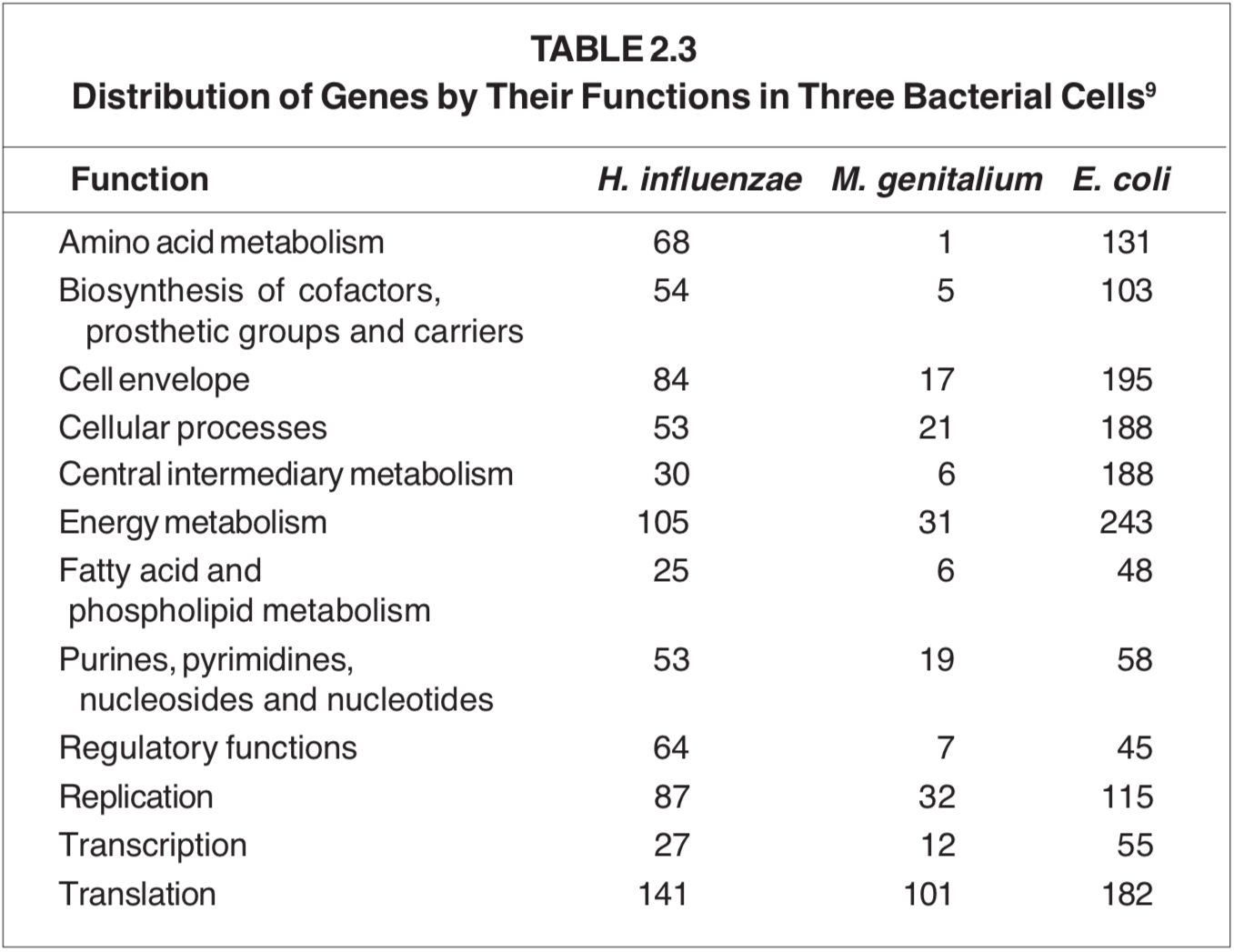
From this table it is seen that E. coli requires more than 1500 different proteins for growth. Most of these proteins are biocatalysts — “enzymes” — that promote specific chemical conversions.
As for the biological functions of the other three classes of biopolymers, nucleic acids are the repositories and transmitters of the genetic information; lipids segregate the interior of the cell from its environment and, along with polysaccharides, serve as energy reserves. In microorganisms, polysaccharides also constitute part of the cell’s outer envelope.
The non-polymeric, small metabolites are only a small portion of the cell by weight, but their presence is absolutely essential for life-processes. In fact, it is the chemical transformations of these compounds that make life possible. (This topic is explored further in the next chapter.) Metabolic intermediates represent transitional substances between precursors and building blocks. Building blocks are used, of course, to make the all-important biopolymers. These in turn are assembled into more complex supramolecular assemblies and organelles.
Matter is organized into successively more complex hierarchies in cells. The logical interdependency among cellular components in the vertical hierarchy parallels nicely the logical ties that connect letters with words, words with sentences, sentences with paragraphs, etc., all the way to the level of a completed manuscript. This concept is illustrated in Figure 2.1.
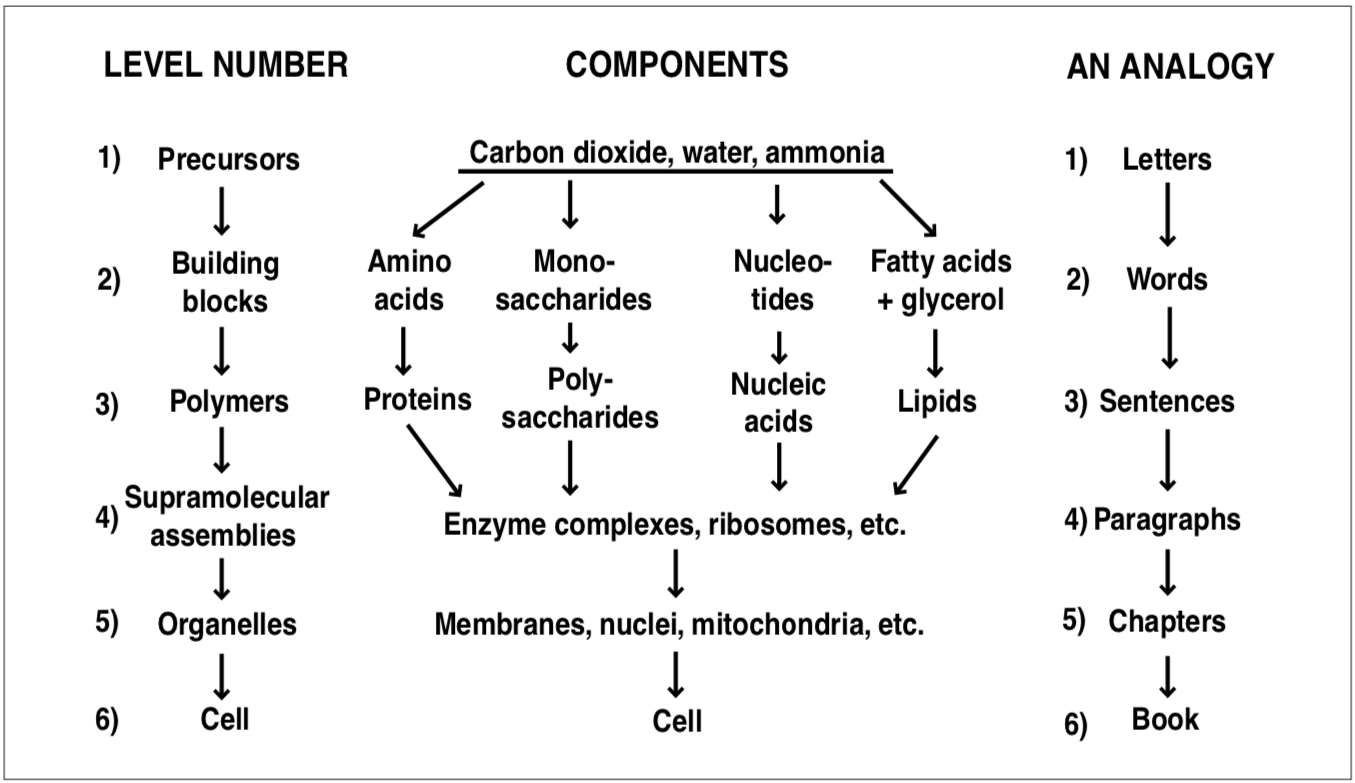
FIGURE 2.1. Organization of matter in the cell.
A crucial difference between biomatter and written material, however, is that the degree of tolerance for error is much smaller in biology. If words are misspelled, sentences are garbled, paragraphs do not hang together, or even if entire chapters are missing from a manuscript, the document may still be partially useful. But given the tight functional interdependence of its components from precursors and biopolymers, cells are in trouble with less than a full complement of all their parts. Each living cell contains thousands of different kinds of substances, present in multiple copies, and sequestered, in the case of a bacterium, in a volume of a few cubic micrometers (see Table 2.1).
At the level of supramolecular assemblies and above, the various strands in Figure 2.1, which represent classes of biopolymers, are intertwined into increasingly complex entities. The biological information in the genetic material and in the many protein molecules becomes a coherent whole somewhat the way paragraphs support each other in a good story. The “story” in this case is the life of the cell.
Besides vertical interdependence, there is also a horizontal complementation among the components. One illustration of this interdependence is that the manufacture of proteins requires nucleic acids and, conversely, nucleic acids cannot be made without proteins. This circular relationship between proteins and nucleic acids from a chemical evolutionary viewpoint resembles the classic “chicken and egg” problem.
SUMMARY OF CHAPTER 2
- Although there are many differences between living organisms and inanimate matter, in an unfamiliar setting it may be difficult to distinguish between them. On Earth the lines of demarcation between living organisms and inanimate matter are clear.
- The chemical makeup of living matter consists of large amounts of biopolymers and smaller amounts of metabolites in an aqueous setting.
- Living matter is organized into hierarchies, with the components being organizationally interdependent in both the vertical and the horizontal dimensions.
REFERENCES AND NOTES ON CHAPTER 2
1. Weiss PA. 1973. A random walk in science. London and Bristol: The Institute of Physics; NY: Crane, Russel and Co., p 124-136.
2. Biemann K, Oro J, Toulmin P (III), Orgel LE, Nier AO, Anderson DM, Simmonds PG, Flory D, Diaz AV, Rushneck DR, Biller JA. 1976. Search for organic and volatile inorganic compounds in two surface samples from the Chryse Planitia region of Mars. Science 194:72-76.
3. These numbers are based on data from: Neidhardt FC, editor. 1966. Escherichia coli and Salmonella. Washington DC: ASM Press, p 14.
4. The availability of each type of atom is different; some are very abundant on Earth, while others are quite scarce. Out of every 100 atoms on Earth, 62 are oxygen, 21 are silicon, 7 are sodium, and 2 are iron. The remaining 8 atoms may be any of the other elements. To find a carbon atom, for instance, we would have to sort through about 8000 atoms.
In contrast, among atoms found in living matter, out of every 100, 60 are hydrogen, 26 are oxygen, and 11 are carbon. Thus it is clear that the atomic content of living matter does not reflect the general composition of Earth.
5. Szent-Gyorgyi A. 1972. The living state. NY: Academic Press, p 8.
6. A curious fact about nearly all “building-block” type substances is that they are structurally asymmetric, i.e., these substances do not have an axis of symmetry. As such, each molecule has a non-biological “twin” which has identical atomic composition to the biologically important subunit, except that the atoms are arranged backwards, and the two structures are spatially mirror images of each other.
Asymmetric substances are similar to our left and right hands, which are non-symmetric mirror images of each other. When a chemical process is used in the laboratory to produce one of these asymmetric substances, the outcome is invariably a fifty-fifty mix of the substance and its mirror image.
Biological systems, on the other hand, are able to produce these asymmetric substances without the contaminating presence of their mirror images. This is accomplished with the help of biological catalysts, called enzymes.
7. The number of possible different sequences for a 100-amino-acid-long protein is 1.2x10130, or 12 followed by 129 zeros!
8. The function of proteins frequently depends on their three-dimensional shapes. The order in which amino acids are linked together influences the protein’s shape immensely.
9. The information in Table 2.3 was compiled from three articles: (a) Fraser CM, Gocayne JD, White O, Adams MD, Clayton RA, Fleischmann RD, Bult CJ, Kerlavage AR, Sutton G, Kelley JM, et al. 1995. The minimal gene complement of Mycoplasma genitalium [see comments]. Science 270:397-403; (b) Fleischmann RD, Adams MD, White O, Clayton RA, Kirkness EF, Kerlavage AR, Bult CJ, Tomb JF, Dougherty BA, Merrick JM, et al. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd [see comments]. Science 269:496-512; and (c) Blattner FR, Plunkett G (III), Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, et al. 1997. The complete genome sequence of Escherichia coli K-12 [comment]. Science 277:1453-1474.
In this table the only genes listed are those whose functions have been identified, at least in a preliminary fashion. For each organism, only 50-60% of the genes are accounted for.