©Copyright 2018 GEOSCIENCE RESEARCH INSTITUTE
11060 Campus Street • Loma Linda, California 92350 • 909-558-4548

OXYGEN AND EVOLUTION
by
G. E. Snow
Associate Professor of Biology,
Andrews University
and
G.T. Javor
Associate Professor of Chemistry,
Andrews University
Related page —|REACTION|
Evolutionary theory proposes a chemically reducing atmosphere during the early history of this earth. This is considered necessary for the production and survival of many necessary compounds associated with life. Some recent data raises serious questions regarding the plausibility of such a model. The authors discuss some of this evidence.
When we take a breath of air, we do it for the purpose of providing oxygen to our body tissues. Without the continuous supply of this gas neither we nor the great majority of organisms on the earth could exist for more than a few minutes. It may come as a surprise then to learn that oxygen is potentially poisonous to all life forms [2].
During the normal course of metabolism in living tissues, oxygen may combine with protons (H+) and/or electrons (e-) to form a super-oxide radical (O2-) or a hydroxyl radical (OH) or a molecule of hydrogen peroxide (H2O2). Any of these products of oxygen cause havoc in the organism by significantly modifying the structures of the molecules that participate in the chemical reactions of life. Fortunately in all oxygen-using organisms we find elaborate enzymatic systems operating which render the toxic products of oxygen harmless.
A relatively small number of species do not have this enzymatic system to protect themselves from the toxic products of oxygen. Such organisms, called anaerobes, can only exist in the absence of oxygen, for simple exposure to air quickly kills them. Anaerobic organisms, as a rule, are simpler in structure than the oxygen-requiring ones and therefore in the evolutionary model they are thought to be most like the first organisms on earth. As a logical corollary, evolutionists postulate the existence of an oxygen-free atmosphere on the primitive earth. This primordial atmosphere would have consisted of mainly hydrogen, ammonia, methane and water vapor. In contrast, our present atmosphere contains mostly oxygen (21%) and nitrogen (78%).
Although Pasteur's work in the last century gave generally accepted evidence that life could not arise spontaneously from non-living sources under current environmental conditions, by the middle of this century the topic of spontaneous generation of life once more became one of major interest. In the last 25 years a number of laboratories throughout the world have been engaged in experiments to produce components of living cells under "primitive earth" conditions.
A measure of success has been achieved by these workers. Biologically significant substances, such as amino acids (the building blocks of proteins), purines and pyrimidines (some of the building blocks of nucleic acids), certain vitamins and simple sugars have been synthesized under postulated "primitive earth" conditions. However, in all successful experiments free oxygen was absent. When oxygen was present, no biologically significant substances were formed [3].
Currently evolutionists assume that free oxygen was all but absent during a significant portion of the earth's 4.5 billion year history. It was during this oxygen-free period that the first life forms were thought to evolve. Then, with the emergence of photosynthetic plants, free oxygen began to be released into the atmosphere as a by-product of photosynthesis, until the present atmospheric level of this gas was reached [4], [5].
Photosynthesis may be represented by the equation:
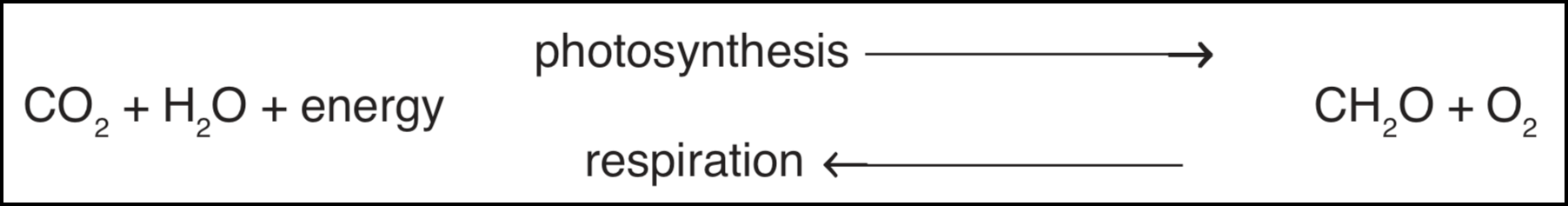
Much of the oxygen produced during photosynthesis is used up during respiration by animals, decomposers and the plants themselves to yield carbon dioxide and water once more. The only net gain to the atmosphere in oxygen is proportional to the amount of reduced carbon (CH2O) not used up in respiration (see equation above). This remaining reduced carbon in plant material will eventually be reoxidized to carbon dioxide and water except for that which is buried in the crust of the earth. The quantity of this buried material can serve to approximate the net gain in atmospheric oxygen which could have been produced by photosynthesis. Current estimates of the mass of organic carbon in sedimentary rocks is 6.8×1021 grams [6]. Assuming that all of this carbon was in the form of CO2 prior to photosynthesis, we can account for the existence of 18.2×1011 grams of oxygen, which is about 15 times more than what there is in our atmosphere at present. The excess amount has presumably been absorbed by the "oxygen sink" processes, such as the oxidation of iron, sulfur and volcanic gases. It would thus appear that the above-presented evolutionary scenario is based on sound scientific reasoning.
Additional considerations of the natural processes involved, however, challenge the validity of this evolutionary scheme. Dr. Van Valen, a member of the committee on evolutionary biology at the University of Chicago, questions the notion of the slow build-up of oxygen in our atmosphere [7]. He indicates that photosynthesis by green plants may be an inadequate explanation for the early accumulation of oxygen. According to him the net production of oxygen today and throughout Phanerozoic time (0.6 billion years), is about equal to that absorbed by the continuous "oxygen sink" processes. How could there be any net oxygen accumulation in the atmosphere during an earlier period of presumably much less photosynthesis and a larger "oxygen sink"?
Van Valen postulated several possible solutions to this problem, none of which were to his liking, and concluded: "... the cause of the original rise in oxygen concentration presents a serious and unresolved quantitative problem" [7].
Dr. Carruthers of the Naval Space Research Laboratory in Washington, D.C. pointed out an additional difficulty with the initial rise in atmospheric oxygen by green plant photosynthesis. An atmosphere void of oxygen would not contain the ultraviolet-absorbing ozone layer. Any photosynthesizing organism, by definition, would be exposed to light radiation and doubtless would be destroyed by the lethal short wavelength ultraviolet rays [8].
Ultraviolet radiation, on the other hand, plays an important role in the production of atmospheric oxygen. It has been known for some time that in the earth's upper atmosphere, above the ozone layer, molecules of water are shattered by the strong ultraviolet radiation of the sun.
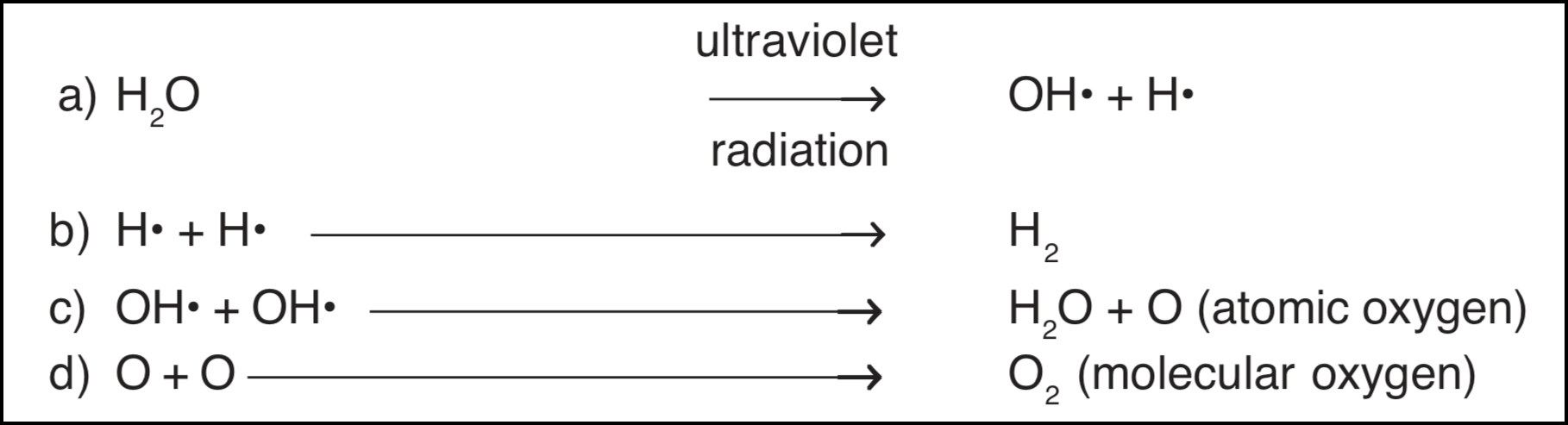
The eventual products of this reaction, as indicated above, are atomic and molecular oxygen and hydrogen. Hydrogen being lighter than air escapes the earth's atmosphere while oxygen remains.
Calculations for the production of oxygen by the photodissociation of water vapor were made by Dr. Brinkman of the California Institute of Technology, using certain assumptions where data was not available. He found that this process could produce 32 times the amount of oxygen currently found in our atmosphere and that a minimum of one fourth of this atmospheric level of oxygen should have been present for more than ninety-nine percent of this earth's history [9].
These results were awarded a mixed reception, because of their unfavorable implications for current evolutionary postulates. Then, pictures taken by a special camera placed on the surface of the moon during the Apollo 16 mission revealed that substantial amounts of hydrogen are leaving the earth's atmosphere, due to the action of ultraviolet radiation on the water vapors of the upper atmosphere [10]. This finding shows that the photodissociation of water is a significant physical reality and an important source of atmospheric oxygen [11]. Dr. Carruthers, who directed these experiments during the Apollo 16 mission, cites a presently lower rate of oxygen production than Dr. Brinkman (about 10 times lower), but indicates that in the past these rates could have been several times greater [8].
More recently, the Mariner 10 spacecraft flew by the planet Venus and radioed back to earth information about the composition of its upper atmosphere. Unexpectedly, the atomic oxygen (O) content of the upper atmosphere of Venus was found to be similar to what it is on earth [12]. Since it is very unlikely that oxygen is being produced on Venus by photosynthesis in plants, it follows then that it must be produced by the photodissociation of water vapor [9].
All available evidence taken together seems to indicate that it is no longer tenable to postulate the existence of long periods of an oxygen-free atmosphere at anytime during the earth's history. But the presence of oxygen in the atmosphere rules out the possibility of any biologically significant compounds being formed in the "primitive atmosphere." This realization has forced some scientists to propose that biological building block substances such as amino acids were actually brought to earth by meteorites [13]. This amounts to admitting their inability to postulate a scientifically valid mechanism, which could yield even the simplest building blocks of biologically important polymers in the context of chemical evolution.
The concept of spontaneous generation of life is the only logical alternative to the Biblical account of creation. Evolutionists, rejecting the Mosaic account of our origins as a myth, have enthusiastically advocated this other alternative. They have turned to the book of nature to gain support for their concepts. But "... the book of nature and the book of revelation bear the impress of the same master mind, they cannot but speak in harmony. By different methods and in different languages, they witness the same great truths" [14].
The validity of this statement is apparent when we consider the origins of atmospheric oxygen and the chances for the spontaneous generation of life. The book of nature tells us that if oxygen has always been in the atmosphere of our earth, then life could not come about by a slow step-by-step self-organization of matter, but rather through a creative act by the One who commanded that "... the earth bring forth living creatures after their kind" [15].
REFERENCES
[1]See also Javor, G. T. and Snow, G. E. 1974. The Apollo Sixteen mission and biochemical evolution. Review and Herald, March 14, 1974.
[2]Fridovich, Irwin. 1975. Oxygen: boon and bane. American Scientist 63(1):54-59.
[3]Lemmon, R. M. 1970. Chemical evolution. Chemical Reviews 70:95-109.
[4]Rutten, M. G. 1971. The origin of life. Elsevier, Amsterdam.
[5]Miller, Stanley L. and Orgel, Leslie E. 1974. The origins of life on the earth. Prentice-Hall, Inc., New Jersey.
[6]Rubey, William W. 1951. Geologic history of sea water: an attempt to state the problem. Bulletin of the Geological Society of America 62:1111-1148.
[7]Van Valen, Leigh. 1971. The history and stability of atmospheric oxygen. Science 171:439-443.
[8]Carruthers, G. R. 1973. The hydrogen geocorona, and the problem of the origin of the atmospheric oxygen. Presented at the meeting of the American Chemical Society, University of Delaware, April 18, 1973.
[9]Brinkman, R. T. 1969. Dissociation of water vapor and evolution of oxygen in the terrestrial atmosphere. Journal of Geophysical Research 74:5355-5368.
[10]Carruthers, G. R. and Page, T. 1972. Apollo 16 far-ultraviolet camera-spectrograph: earth observations. Science 177:788-791.
[11]News Release #30-72-7 from the Naval Research Laboratory, Washington, D.C.
[12]Broadfoot, A. L. et al. 1974. Ultraviolet observations of Venus from Mariner 10: preliminary results. Science 183:1315-1318.
[13]Life on earth: from chemicals in space? Chemical and Engineering News, November 19, 1973, pp. 21-22.
[14]White, E. G. 1952. Education. Pacific Press Publishing Association, Mountain View, California, p. 128.
[15]Genesis, chapter one.